- Fierce JPM Week: How 23andMe plans to harness its massive database to further its ambitions in drug development (fiercehealthcare.com)
Drug development is expensive, can take years to complete and doesn't guarantee a novel therapy for treating disease...But consumer genetic testing company 23andMe is forging ahead to leverage its massive database of genetic data for research and to make and sell its own therapies...Using genetic data based on the company's 12 million genotype customers and billions of phenotypic data points gives 23andMe a distinct competitive advantage for its therapeutics business, Kenneth Hillan, 23andMe’s head of therapeutics, said..."We talk about it as our secret power. It really is that statistical power, the size and scale of our databases, and using genetics to see things in terms of drug targets within diseases that others just can't see," he said...READ MORE
- COVID vaccines help Pfizer, Moderna reign supreme reputation-wise, but they’re a double-edged sword for J&J and AZ (fiercepharma.com)
Pfizer and Moderna are now two of the leading pharma companies in the U.S. when it comes to public image, thanks to their COVID vaccines. On the flip side, vaccine rivals J&J and AstraZeneca have seen their reputations plummet...This is according to a new survey by market research firm Leger, which looked into how the pharma industry’s reputation has changed during COVID and the creation of new vaccines against the disease...READ MORE
- Fourth Scientist Pleads Guilty to Stealing GSK Information for China (biospace.com)
Ex-GlaxoSmithKline researcher Lucy Xi has pled guilty to conspiracy to steal trade secrets from her former company to help a rival firm launch a business in China. She is the fourth person to plead guilty to the offense...Xi and co-defendants Yan Mei, Yu Xue, Yan Mei, and Tao Li created Renopharma in the guise of conducting research and development of anti-cancer therapies. However, it was found that Renopharma had been operating as a repository of stolen GSK information and was receiving compensation and subsidies from the People's Republic of China to do so...READ MORE
- Pill for treating COVID at home comes to Nevada, but in short supply (reviewjournal.com)
The first pill authorized in the U.S. for treating COVID-19 at home will initially be offered in Nevada primarily to patients in long-term care and skilled nursing facilities because of scarce supplies...The remainder, less than 10 percent of the state’s initial allotment of Pfizer’s antiviral medication Paxlovid, has been given to University Medical Center in Las Vegas and to Renown Regional Medical Center in Reno, members of the Nevada State Board of Pharmacy said...The drug’s authorization is “hands down, next to the vaccine, the most significant milestone in the pandemic,” said Dr. Shadaba Asad, the medical director of infectious disease at UMC...Supplies of the Pfizer pill currently are extremely limited across the country. Nevada’s initial supplies are enough to treat 480 patients, with allocations expected to grow as production ramps up, pharmacy board executive secretary David Wuest said...READMORE
- Nearly 4K people may have gotten slightly lower Pfizer COVID-19 dose at a Kaiser Permanente location (fiercehealthcare.com)
Nearly 4,000 people may have received a slightly less than recommended dose of the Pfizer COVID-19 vaccine at a Kaiser Permanente location in California last year. The health system is offering repeat vaccinations to anyone affected...The vaccinations were given at the Kaiser Permanente Walnut Creek Medical Center...Individuals may have received between 0.01 and 0.04 milliliters less than the correct 0.3-ml dose. The health system reportedly said the incident was a misunderstanding among staff, which it has retrained, and is contacting patients about the error...READ MORE
- GlaxoSmithKline rushes to accelerate COVID-19 antibody output amid omicron-driven demand (fiercepharma.com)
GlaxoSmithKline and Vir Biotechnology are rushing to speed up production of their COVID-19 therapy, now that they're the only companies with an antibody that appears to be truly effective against omicron...The FDA...cleared a Samsung Biologics site as a second manufacturing facility to make GSK and Vir’s Xevudy (sotrovimab)...Along with adding the new facility, GSK and Vir worked with external partners to secure additional batches of drug substance to support supply this year...READ MORE
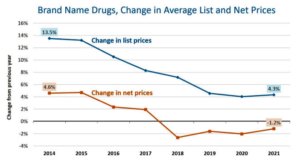
- Tales of the Unsurprised: Brand-Name Drug Prices Fell for the Fourth Consecutive Year (drugchannels.net)
Reality has again failed to cooperate with the politically motivated cries of “skyrocketing drug prices” or anecdotes about companies “jacking up prices” (as President Biden recently claimed)...Brand-name drug prices continue to decline, while the prices of other healthcare products and services continue to rise. For 2021, brand-name drugs’ net prices dropped for the fourth consecutive year. Meanwhile, brand-name drug list prices grew more slowly than overall inflation. What’s more, we project that the gross-to-net bubble for patent-protected brand-name drugs will exceed $200 billion in 2021...The factors that drive declining brand-name drug prices remain for 2022, suggesting that these trends will continue...READ MORE
- Judge Grants Stay in Federal Case Against Pharmacy DIR Fees Until Proposed Rule Finalized (ncpa.org)APhA, others file federal lawsuit against HHS to close DIR loophole (pharmacytoday.org)
The National Community Pharmacists Association and the American Pharmacists Association... issued the following statement in response to a decision by the U.S. District Court for the District of Columbia to grant a stay the case, NCPA v. Becerra, which challenges the legality of retroactive pharmacy price concessions, also known as pharmacy direct and indirect remuneration fees:...“We are pleased by the court’s decision to grant our request for a stay, or a pause in the litigation, until the recently proposed rule potentially addressing retroactive pharmacy DIR fees is finalized. We are currently analyzing the proposed rule to determine whether it addresses our longstanding concerns with retroactive pharmacy DIR fees, and we plan to submit comments reflecting our analysis. Since our litigation also seeks to end retroactive pharmacy DIR fees, we believe, and the court agreed, that a pause in the case is appropriate pending the outcome of the rulemaking process.”
- CMS pulls Trump-era Most Favored Nation drug price model (fiercehealthcare.com)
The Biden administration has officially pulled a controversial model that would have tied prices for drugs reimbursed under Part B to prices paid by countries overseas...The Centers for Medicare & Medicaid Services issued a final rule last week that pulls the demonstration approved at the tail end of the Trump administration. Providers had slammed the Most Favored Nation model due to concerns over reimbursement...CMS said in the final rule published on Dec. 29 that the model sparked four lawsuits from the drug industry, which resulted in a legal stay delaying it from going into effect...READ MORE
- Attorney General Aaron Ford announces Nevada to join opiod settlement (reviewjournal.com)Nevada to receive $285 million in latest round of opioid settlements (thenevadaindependent.com)
Attorney General Aaron Ford announced Thursday that Nevada would join a multi-state opiod settlement with drugmakers and distributors...Ford said that the state would receive around $285 million through a pair of settlements...Last year, Ford announced a $45 million settlement against one company involved in the opioid litigation. The lawsuit is being handled on a contingency fee basis for the state by Eglet Prince, the law firm where Ford worked as a private attorney before being elected attorney general in 2018. Ford, however, recused himself from the selection process...Ford in August announced that Nevada would opt out of a $26 billion multi-state settlement...READ MORE