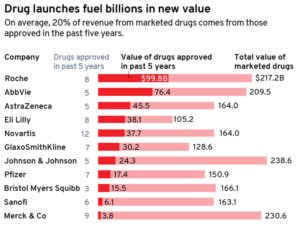
- Who’s getting the most out of their R&D engine? Pharma’s top 11, ranked (fiercepharma.com)
Drugmakers have myriad tools in their arsenal when looking to grow sales. They can acquire marketed drugs, raise prices or focus on growing the reach of their existing medicines. But it's often new drug approvals that reign supreme and ultimately prove the worth of a company's development engine...covered in a recent Evaluate Vantage report, the team at Fierce Pharma took a close look at the recent approvals for 11 of the world's biggest drugmakers by revenue. Specifically, we're highlighting the dollar value of the industry's launches from the last five years and analyzing how the new meds fit into each company's overall portfolio...READ MORE
- Is NICE becoming nicer? England’s cost-effectiveness watchdog lays out plans to speed access to new medicines (fiercepharma.com)
England's cost-effectiveness watchdog, typically a fierce critic of pharma's launch prices, says it’s revamping the way it reviews new drugs, devices, diagnostics and more in a push to provide greater access, sooner...The National Institute for Health and Care Excellence, or NICE, rolled out its planned remodel...aimed at providing “faster, fairer” access to the drugs and devices it reviews for National Health Services (NHS) patients...READ MORE
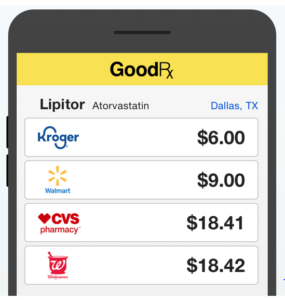
- GoodRx partners with Surescripts to provide real-time drug discount pricing (drugstorenews.com)
GoodRx has entered into an agreement with Surescripts, to deliver drug discount price information to prescribers using Surescripts Real-Time Prescription Benefit when prescribing medications for uninsured patients and patients whose price information isn’t already available from their PBM or health plan...By improving price transparency at the point of care, prescribers can have more informed conversations with their patients and ensure they can afford the treatment they need before getting to the pharmacy counter...READ MORE
- Pfizer, Moderna and Alnylam flag pharma labor shortage in Massachusetts—and the people bottleneck doesn’t stop there (fiercepharma.com)
Raw materials and limits on high-tech equipment often take center stage when it comes to discussions around manufacturing bottlenecks. But the COVID-19 pandemic has exposed another weak link in the pharmaceutical supply chain: people...As COVID-19 vaccine production moves full-tilt, mRNA players Pfizer and Moderna are having trouble recruiting talent...Hiring challenges, which have been exacerbated by the pandemic, aren’t unique to COVID-19 vaccine makers...READ MORE
- Pfizer, BioNTech score FDA’s first full COVID-19 vaccine nod, quickly triggering stricter mandates (fiercepharma.com)
The U.S. FDA has awarded the first full approval for a COVID-19 vaccine to Pfizer and its German partner BioNTech, a historic decision that comes weeks ahead of its previously expected Labor Day deadline...Pfizer’s jab, now approved for people aged 16 and older, will remain under an emergency nod for adolescents aged 12 to 15...The agency’s full approval for Pfizer’s mRNA shot, now marketed as Comirnaty, is expected to spark a wave of vaccine mandates from companies, universities and organizations awaiting the agency’s final sign-off...READ MORE
- FDA set to issue full approval for Pfizer vaccine on Monday (msn.com)
The New York Times reports that the Pfizer shot will be the first of the coronavirus jabs to be cleared by the FDA...According to the report, the FDA originally had planned to approve the vaccine before Labor Day, but decided to accelerate its ruling...The full approval of the shot also paves the way for employers and private companies to mandate employees and patrons to be vaccinated...READ MORE
- New database could accelerate drug repurposing for various diseases (worldpharmanews.com)
Researchers have created a new open-access database of information on drug candidates and how they are metabolised by the body, which could help speed up the repurposing of old drugs as new treatments...the process of developing new drugs is costly, can take decades, and often leads to failed treatments. The database, called NICEdrug.ch and described today in eLife, may help expedite the process by helping scientists find promising, existing drugs that might be repurposed...READ MORE
- Most Americans Oppose Biden’s Prescription Drug Price Controls (realclearhealth.com)
In response to U.S. prescription drug spending rapidly outpacing inflation, reaching an estimated $358.7 billion in 2020, President Biden last week demanded that Congress adopt strict federal price controls on prescription drugs...to cap annual out-of-pocket drug spending for Medicare beneficiaries, empowering the Secretary of Health and Human Services to choose pharmaceutical winners and losers, and financially crippling penalties for pharmaceutical companies that don’t acquiesce to price controls — are likely to have disastrous effects on the availability of critical medications. What’s more, they’re deeply unpopular with the overwhelming majority of Americans, and could prove to be a significant fiscal and health liability for our nation in the years to come...READ MORE
- Despite pharma’s pandemic push, consumer trust in the industry still comes down to costs: study (fiercepharma.com)
A new study from Accenture shows a boomerang back to a familiar topic—drug costs...“We asked what would increase trust in the pharmaceutical industry, and for many people there’s a clear link between cost and trust,”...The top two answers specifically pointed to reduced medication costs (41%) and more transparency in pricing (39%) as measures that would make the respondents trust pharma companies more...An earlier Accenture report from May suggested the new economic reality for pharma meant companies would have to work at changing the cost narrative with customers. It suggests focusing on value and outcome-based pricing to build trust...READ MORE
- Prescription Drug Monitoring Programs: Effects on Opioid Prescribing and Drug Overdose Mortality (reason.org)PRESCRIPTION DRUG MONITORING PROGRAMS: PDMP EFFECTS ON OPIOID PRESCRIBING AND DRUG OVERDOSE MORTALITY (reason.org)
The Centers for Disease Control and Prevention reported 70,630 drug overdose deaths for 2019 in the United States, 70.5 percent of which were opioid-related...Prescription Drug Monitoring Programs are the most popular interventions states enact to address opioid addiction and overdoses...This study finds that, although Prescription Drug Monitoring Programs’ intermediary purpose to reduce prescribing has been achieved by reducing opioid distribution by 7.7 percent, they have had inconsistent effects on prescription opioid overdoses, while increasing total opioid overdoses by 17.5 percent due to increased mortality from the black market varieties by 19.8 percent...READ MORE