- Don’t let drug companies run Nevada’s health care industry (thenevadaindependent.com)Medication prices could be capped under proposed Nevada bill (reviewjournal.com)
Members of both political parties agree that keeping life-saving prescription drug prices reasonable saves lives; however, there is an ongoing debate about the best way to accomplish this goal...For many Democrats, government-imposed price controls are the solution. This past legislative session, they passed Assembly Bill 250, which would prevent the major drug manufacturers from inflating the cost of our prescription drugs by having the government set these products’ prices...Republicans, on the other hand, argue that promoting marketplace competition is a more effective solution than more government. That’s why Gov. Joe Lombardo rightly vetoed this bill...Regardless of where one falls on the political spectrum, everyone in this state should agree that regulating away the private market entities that help restrain the drug companies’ ability to rig prices higher shouldn’t be the answer. And yet, that’s exactly what the drugmakers are telling them to do...READ MORE
- National cancer group reports widespread chemo shortages, calls on government and industry to help resolve them (fiercepharma.com)
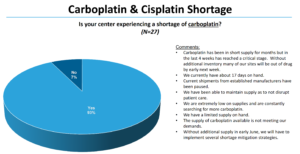
As pharma supply chain problems drag on, a shortage of key cancer drugs has afflicted a large number of treatment centers and many patients. Now, a leading treatment center group is putting more statistics behind the shortage...Late last month, the National Comprehensive Cancer Network Best Practices Committee conducted a survey (PDF) of 27 member centers across the U.S. The group found that nearly all treatment centers, or 93%, reported a carboplatin shortage. In addition, 70% of the centers reported a cisplatin shortage...READ MORE
- FDA, weighing Perrigo’s OTC birth control application, raises questions about real-world use (fiercepharma.com)
As a long-awaited advisory committee meeting on Perrigo’s over-the-counter birth control prospect Opill nears, the FDA says many big questions remain. Chief among them: Will people use the drug as intended in the real world?...Ahead of this week’s joint expert panel meeting, the FDA released briefing documents posing two main questions for its expert committees: First, just how likely are consumers to use Opill “in an effective and safe manner,” relying solely on the nonprescription prospect’s label and without help from a healthcare professional?...Second, will consumers who shouldn’t use the product avoid the temptation?...READ MORE
- Supreme Court maintains access to abortion pill, blocking restrictions on its use (biopharmadive.com)
The stay suspends an order by a Texas judge that had invalidated the FDA’s approval of mifepristone, keeping it available while a circuit court hears the case...The Supreme Court on Friday allowed the abortion pill mifepristone to remain on the market for the time being, suspending a lower court order that would have curtailed its availability in the U.S....The 7-2 ruling stays a verdict earlier this month by U.S. District Court Judge Matthew Kacsmaryk invalidating the Food and Drug Administration’s 2000 approval of mifepristone. Justices Samuel Alito and Clarence Thomas dissented...READ MORE
- Grail sued by 3 women over alleged ‘frat house’ culture, harassment and retaliation (fiercebiotech.com)
Three women are suing the cancer blood test developer Grail claiming that, during their time as high-level sales employees, they suffered harassment, discrimination and ultimately retaliation under “a fraternity house” type of toxic work culture led by senior staff...the three separate lawsuits said that Grail failed to properly respond to the plaintiffs' complaints of a “sexually charged” and hostile work environment, including racist remarks made by a coworker in one case. They also each claim they were denied equal pay under California law...READ MORE
- U.S. drug shortages highlight dependence on China, gray supply chains (thechinaproject.com)
The U.S. Food and Drug Administration is loosening restrictions to allow the Chinese company Qilu Pharmaceuticals to import cisplatin, a cancer medicine currently in short supply...The emergency move to import Qilu’s cisplatin, which is not FDA-approved, comes as U.S. hospitals ration chemotherapy drugs that can dramatically improve a patient’s prognosis. An FDA official told The China Project that the agency is exploring continued importation of cisplatin and temporary importation of another cancer drug, carboplatin, but, when asked, wouldn’t provide details on plans for further temporary importation from China. This is the first time the U.S. has allowed for temporary importation of cisplatin, the FDA official said...READ MORE
- How Discount Cards Work: A Primer on GoodRx and Its Competitors (video) (drugchannels.net)
In my most recent video webinar, I explored how the rapid expansion of patient-paid prescriptions—via cash-pay pharmacies and discount card vendors—is transforming the prescription market...Below, I follow the dollar when a patient uses a discount card to pay for a generic drug prescription...As you’ll see, a discount card can save money for consumers by leveraging several quirks of U.S. retail pharmacy pricing. Such cards also enable novel profit streams for both pharmacy benefit managers (PBMs) and the card vendors themselves...READ MORE
- Walgreens inks another deal for clinical trials business as CVS exits research recruitment (fiercehealthcare.com)
Retail pharmacy giant Walgreens inked another partnership to recruit participants for research as it continues to build out its clinical trials business...The company signed a deal with biotech startup Freenome to advance clinical trials of its blood-based tests for the early detection of cancer...It marks the sixth contract that Walgreens has publicly disclosed for its year-old clinical trials business unit. The pharmacy chain launched the unit back in June 2022 as the company's healthcare ambitions continue to grow...While Walgreens continues to grow the business, rival CVS Health announced in May it was winding down its clinical trials arm just two years after its launch. The company will fully exit clinical trials by the end of 2024...READ MORE
- Ahead of high-stakes California trial, GSK notches Zantac win in Canada (fiercepharma.com)GSK was warned repeatedly about Zantac impurity but played down risks: Bloomberg (fiercepharma.com)
As GSK's July court date nears for a key Zantac trial in California, the company can wipe its hands of at least one Canadian class action suit...The company said in a Friday statement that it “welcomes the decision” of the British Columbia Supreme Court to dismiss a proposed class action suit on behalf of Canadian Zantac users...A Vancouver man filed the lawsuit in 2020, alleging that his use of the heartburn med from 2018 to 2019 caused him to develop cancer. His complaint named more than a dozen companies as defendants, including Sandoz Canada and GSK...But the court dismissed the case due to “the uncontroverted evidence that neither ranitidine nor NDMA are reliably associated with increased cancer risk,” GSK said in its statement...READ MORE
- Biotech layoffs gather pace as industry downturn persists (biopharmadive.com)
More than 5,000 employees have been let go from biotech and pharmaceutical companies so far this year...At least five dozen biotechnology companies have laid off employees so far this year in a sector-wide contraction that has reached large and small drugmakers alike...Brought on by enduring funding challenges, the consolidation has resulted in roughly 3,200 biotech employees losing their jobs between Jan. 1 and early April...While the workforce reductions aren’t a new development — more than 100 biotech companies conducted layoffs last year — the pace of announcements has accelerated, suggesting the industry hasn’t recovered from 2022’s market downturn...READ MORE