- As Drug Prices Keep Rising, State Lawmakers Propose Tough New Bills to Curb Them (khn.org)
Fed up with a lack of federal action to lower prescription drug costs, state legislators around the country are pushing bills to penalize drugmakers for unjustified price hikes and to cap payment at much-lower Canadian levels...These bills, sponsored by both Republicans and Democrats in a half-dozen states, are a response to consumers’ intensified demand for action on drug prices as prospects for solutions from Congress remain highly uncertain...READ MORE
- Fresenius Kabi unit admits it hid records from FDA inspectors—and settles with DOJ for $50M (fiercepharma.com)
Fresenius Kabi Oncology fell afoul of the FDA in 2013 when the agency discovered employees had hidden records before a manufacturing inspection. Now, the drug ingredients manufacturer is admitting fault—and has reached a deal with the U.S. Department of Justice to put the investigation to bed...The Fresenius Kabi unit agreed to pay a $30 million fine, forfeit another $20 million and plead guilty to concealing and destroying records ahead of a 2013 FDA inspection in Kalyani, India...READ MORE
- Nevada climbs out of bottom in administering vaccine, CDC says (reviewjournal.com)
Nevada no longer has one of the worst COVID-19 vaccination rates per capita in the U.S., according to federal data...The Silver State had consistently ranked among the bottom five states at administering vaccine for weeks. It now ranks 12th worst, the Centers for Disease Control and Prevention reported...READ MORE
- AstraZeneca, Oxford race to update COVID-19 vaccine as study flags weak action against variant (fiercepharma.com)
It didn’t take long before a morale boost for AstraZeneca’s COVID-19 vaccine was overshadowed by disappointment over its waned protection against a newly emerged coronavirus variant...A new study has found AZ’s COVID-19 shot offered “minimal protection” against mild to moderate disease caused by the B.1.351 variant, which was first identified in South Africa, the University of Oxford, the original developer of the vaccine, said...READ MORE
- FDA warning letter smacks Allay Pharma for potency problems, API testing issues (fiercepharma.com)
Florida-based drugmaker Allay Pharmaceuticals is in hot water with the FDA after it failed to right the manufacturing wrongs flagged in an inspection last May...FDA slapped Allay for failing to set up proper procedures and process controls for tablet manufacturing at its plant in Hialeah, Florida, according to a warning letter...Inspectors turned up potency problems, substandard API testing and biologics manufacturing without FDA approval...READ MORE - Pfizer to nearly halve COVID-19 vaccine production timeline, sterile injectables VP says (fiercepharma.com)
DNA production—the first step in Pfizer's vaccine manufacturing process—could soon take just nine to 10 days, rather than 16...With an upsized production goal of 2 billion COVID-19 vaccine doses this year, Pfizer and its German partner BioNTech aren’t resting on their laurels now that their shot, Comirnaty, has emergency nods in the U.S., Europe and beyond. As the companies continue to build out capacity, manufacturing efficiency is getting its own boost...The time it takes the company to produce a COVID-19 vaccine batch could soon be cut from 110 days to an average of just 60...READ MORE
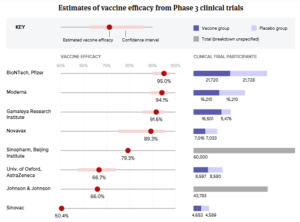
- The first coronavirus vaccines have arrived. Here’s where the rest stand. (biopharmadive.com)
Study results showed vaccines from J&J; and Novavax to be effective against COVID-19. But seemingly weaker protection versus new virus variants have raised concerns...Scientists, drugmakers and governments have moved with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have completed studies proving their vaccines can protect against COVID-19. A half dozen shots from developers in the U.S., U.K., Germany, China and Russia have now been cleared by regulators for emergency use...READ MORE
- PwC joins Medicines Manufacturing Innovation Centre (outsourcing-pharma.com)
CPI, one of the founding partners in the Medicines Manufacturing Innovation Centre, has announced the signing of an agreement with PwC, making the company the latest partner in the collaborative effort. The Centre (established via a partnership among CPI, the University of Strathclyde, UK Research and Innovation, Scottish Enterprise and founding industry partners AstraZeneca and GSK) seeks to promote more innovative drug manufacturing processes and enable a more agile supply chain...PwC and the existing partners will work to maximize technological opportunities across the pharma supply chain. Efforts include...projects, intended to advance emergent and disruptive technologies. The program is partially funded by Innovate UK through the Industrial Strategy Challenge Fund and Scottish Enterprise via the Scottish Government...READ MORE
- AbbVie, Novo Nordisk lead pharma TV advertisers into big-spending January (fiercepharma.com)
Pharma marketers are continuing their TV ad push into 2021. January pharma TV spending picked up where December ended—matching branded ad spending among the top 10 almost dollar for dollar...The highest spenders racked up $216 million for the month after a robust $217 million December, according to data from real-time TV ad tracker iSpot.tv...Now, with the third month in a row of the top 10 clearing $200 million, are pharma companies setting a new standard for TV spending?...Maybe. Five autoimmune disease treatments and three diabetes meds at the top of the list may point to increases in categories' competition. That could, in turn, foster hand-in-hand media buying increases to get TV mindshare...READ MORE
1. Humira
2. Rybelsus
3. Dupixent
4. Skyrizi
5. Ozempic
6. Trulicity
7. Eliquis
8. Xeljanz
9. Enbrel
10. Opdivo and Yervoy - FDA clears Lilly’s COVID-19 antibody cocktail for emergency use (biopharmadive.com)
The Food and Drug Administration...cleared an antibody drug cocktail from Eli Lilly for emergency use for treating people recently diagnosed with COVID-19...The cocktail, a combination of two coronavirus-targeting antibodies, is authorized only for people with mild or moderate symptoms of COVID-19, but who are at high risk of the disease's worst effects due to age, underlying medical conditions or other preexisting conditions...The combination pairs Lilly's bamlanivimab...with another antibody called etesevimab that the drugmaker developed in partnership with China's Junshi Biosciences. Each antibody targets a separate section of the "spike" protein used by the SARS-CoV-2 virus to breach the body's cells — a feature designed to preserve the drug's effectiveness even as the virus mutates...READ MORE