- House committee to subpoena AbbVie over drug pricing (biopharmadive.com)
A congressional oversight committee plans to subpoena AbbVie for documents as part of an investigation into drug pricing... The House Oversight and Reform Committee has been seeking documents related to the pricing of AbbVie's top two drugs, which generated more than $23 billion combined last year...Chairwoman Carolyn Maloney, D-N.Y., said the company's responses have been "particularly poor"...AbbVie is the only one to receive a subpoena thus far, with Maloney saying its "noncompliance stands out as particularly egregious." The drugmaker said it was "surprised and disappointed" by the committee's action...READ MORE
- COVID-19 vaccine developers prepare joint safety pledge: WSJ (reuters.com)Covid-19 Vaccine Developers Prepare Joint Pledge on Safety, Standards (wsj.com)
Several COVID-19 vaccine developers, including Pfizer Inc, Johnson & Johnson and Moderna Inc, plan to issue a public pledge not to seek government approval until their vaccine candidates are proven to be safe and effective...The companies would pledge to adhere to high scientific and ethical standards in the conduct of clinical studies and in their manufacturing processes, the Journal report here said, citing the draft of a joint statement that is still being finalized...The news comes amid rising concerns that political pressure ahead of the Nov. 3 election could weigh on the safety and effectiveness of a potential vaccine for the respiratory illness...READ MORE
- Eli Lilly to halt sales of 340B drugs to contract pharmacies with exception of insulin (fiercehealthcare.com)STATEMENT ON ELI LILLY CUTTING OFF ALL ACCESS TO 340B PRICING THROUGH COMMUNITY-BASED PHARMACIES (340bhealth.org)
Eli Lilly will no longer offer discounted products to 340B contract pharmacies, with the exception of insulin. The drug maker is the second to halt sales to contract pharmacies, following a move by AstraZeneca last month...Roughly one-third of the more than 12,000 340B hospitals use a contract pharmacy to dispense the discounted drugs, according to a 2018 report from the Government Accountability Office...The GAO report found that the Health Resources and Services Administration, which oversees the 340B program, does not fully assess compliance with the program’s prohibition on duplicate discounts for drugs prescribed to both Medicaid and 340B...Pharmaceutical manufacturers have also charged that the discount program has gotten too large and unwieldy...READ MORE
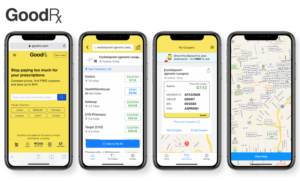
- GoodRx files to go public, boasting track record of profitability (fiercehealthcare.com)How GoodRx Profits from Our Broken Pharmacy Pricing System (drugchannels.net)
Joining a recent spate of digital health companies hitting the public market, GoodRx filed its initial public offering...The startup, which helps consumers find deals on their prescription medications, is looking to raise up to $100 million in an IPO...Unlike many other digital health companies that have gone public, GoodRx has been profitable since 2016...The majority of the company's revenue, or 97%, comes from its prescription offering in which if collects fees from pharmacy benefit managers when consumers use a GoodRx code to fill a prescription...With the acquisition of telemedicine company HeyDoctor...GoodRx...now competes with other startups that combine virtual care and pharmacy services such as Hims, Hers, Ro and Nurx...GoodRx doesn’t sell medications; it instead directs consumers to the drugstore with the best prices and offers coupons...READ MORE
- FDA scolds Mylan for ‘repeated’ manufacturing problems (biopharmadive.com)
The Food and Drug Administration is concerned that generics giant Mylan has deep-rooted problems in its global manufacturing network, with the latest evidence coming from a site in India...The agency posted this week a warning letter about Mylan's Unit 7 facility, which makes dozens of active pharmaceutical ingredients, or APIs, used in blood pressure drugs, antifungals and central nervous system treatments. During a February inspection, regulators found the site did not have adequate procedures in place to prevent contamination. They noted that similar issues were seen last year at Unit 8..."These repeated failures at multiple sites manufacturing API demonstrate that your company's oversight and control over the manufacture of drugs is inadequate. You should immediately and comprehensively assess your company’s global manufacturing operations,"...READ MORE
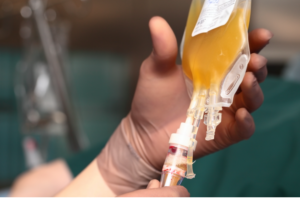
- Dozens of U.S. hospitals poised to defy FDA’s directive on COVID-19 plasma (fiercepharma.com)
Dozens of major hospitals across the U.S. are grappling with whether to ignore a federal decision allowing broader emergency use of blood plasma from recovered COVID-19 patients to treat the disease in favor of dedicating their resources to a gold-standard clinical trial that could help settle the science for good...As many as 45 hospitals from coast to coast have expressed interest in collaborating on a randomized, controlled clinical trial...Officials at some hospitals said they are considering committing only to the clinical trial—and either avoiding or minimizing use of convalescent plasma through an emergency use authorization issued...by the federal Food and Drug Administration...The response comes amid concerns that the Trump administration pressured the FDA into approving broader use of convalescent plasma...A National Institutes of Health panel this week countered the FDA’s decision, saying that the therapy “should not be considered the standard of care for the treatment of patients with COVID-19” and that well-designed trials are needed to determine whether the therapy is helpful. Data so far suggests the treatment could be beneficial, but it’s not definitive...READ MORE
- HHS bids out massive $250M ad campaign to put hopeful spin on coronavirus pandemic: report (fiercepharma.com)
The Department of Health and Human Services plans to spend $250 million in media communications to “defeat despair and inspire hope” around the coronavirus pandemic...an HHS document detailing the project...was sent to communications agencies to solicit bids for what would be an extremely large advertising campaign in the heart of the presidential campaign season...the campaign would tap traditional, digital and social media and partner with the sports and entertainment industries and public health groups “to deliver important public health and economic information the administration can defeat despair, inspire hope and achieve national recovery.”...While it’s not unusual for government agencies to use advertising to promote public health or raise awareness around a consumer issue, the size of the spend and its timing just ahead of the presidential election raised eyebrows....READ MORE
- Costa Rica researchers to trial coronavirus treatment from horse antibodies (reuters.com)Costa Rica researchers to trial coronavirus treatment from horse antibodies (scientificamerican.com)
Researchers in Costa Rica are due to begin trials of an inexpensive coronavirus treatment based on antibodies taken from horses injected with the SARS-Cov-2, the virus that causes COVID-19...Developed by University of Costa Rica’s Clodomiro Picado Institute, the equine antibodies medication is to be tested on 26 patients from mid-September...Costa Rican authorities hope to be able to begin applying the treatment more widely in hospitals if the results from the phase 2 study are encouraging. There are 471 hospitalized coronavirus patients in Costa Rica...Similar efforts are also underway in Argentina and Brazil, while scientists in Belgium are using llamas...READ MORE
- California Rx: State may dive into generic drug market (fiercepharma.com)
California is poised to become the first state to develop its own line of generic drugs, targeting soaring drug prices and stepping into a fiercely competitive drug market dominated by deep-pocketed pharmaceutical companies...The Democratic-controlled legislature overwhelmingly approved a measure...that would direct the state’s top health agency to partner with one or more drug companies by January to make or distribute a broad range of generic or biosimilar drugs — including the diabetes medicine insulin — that are cheaper than brand-name products...The bill, SB-852, also opens the door for California to make its own generic drugs in the future...READ MORE
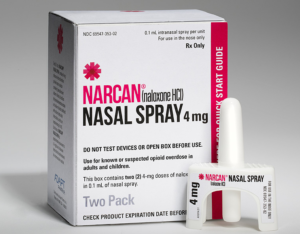
- Narcan must become as commonplace as CPR (thehill.com)Emergent steps to the plate with Major League Baseball and virtual experience for opioid overdose awareness (fiercepharma.com)
While the world continues to navigate the impacts of the COVID-19 pandemic, our other epidemic hasn’t gone away: The rate of opioid overdoses, already a national nightmare, has accelerated since the onset of COVID-19...It’s time to end this continuing tragedy. One of the ways to do this is by making naloxone...a safe, effective and use-specific antidote to opioid overdose — as common among bystanders as CPR...Narcan can be purchased in its generic or branded forms by anyone, without a prescription, at any participating pharmacy...In order to stem our national overdose epidemic, Narcan must be omnipresent in our public places, in our workplaces and schools, and in our homes...Years ago, CPR became a public health imperative. CPR became common after the American Red Cross, the American Heart Association and other major national organizations assumed the task of training. In today’s world, Narcan needs to have similar importance...As we observe International Overdose Awareness Day, it’s time to establish a new public health priority. Surgeon General Jerome Adams, in the first Surgeon General advisory of its kind in the past 15 years, is urging every American to carry Narcan, and be trained to administer it...READ MORE