- Judge wipes out Biogen’s Tecfidera patent protections in suit against Mylan (fiercepharma.com)
In the high-stakes patent fight between Biogen and Mylan over Tecfidera’s main remaining patent, Mylan has scored a major win in federal court...U.S. District Judge...said Mylan “demonstrated by clear and convincing evidence” that certain claims of Biogen’s '514 patent are invalid for “lack of written description.”...The decision threatens Biogen’s bestselling medicine with early generics; Tecfidera, a multiple sclerosis drug, generated $3.3 billion in the U.S. last year. The company's '514 patent is set to expire in 2028, meaning the decision, if upheld, could wipe out years of monopoly sales...Biogen is also facing patent challenges in Delaware federal court. That court's decision will also factor into the ongoing efforts by generics companies to launch copycats...READ MORE
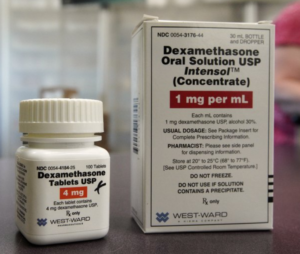
- Cheap drug is first shown to improve COVID-19 survival (apnews.com)
Researchers in England say they have the first evidence that a drug can improve COVID-19 survival: A cheap, widely available steroid reduced deaths by up to one third in severely ill hospitalized patients...The results were announced Tuesday and the British government immediately authorized the drug’s use across the United Kingdom for coronavirus patients like those who did well in the study. Researchers said they would publish results soon in a medical journal, and several independent experts said it’s important to see details to know how much of a difference the drug, dexamethasone, might make and for whom...READ MORE
- America’s global leadership in biopharmaceutical manufacturing (catalyst.phrma.org)
Unfortunately, there are a lot of assertions floating around that tell a misleading story about pharmaceutical manufacturing, diminishing America’s leadership and suggesting our reliance on other countries has put us at risk of potential shortages. In reality...the biopharmaceutical industry supports more than 4 million jobs across the United States, directly employing more than 811,000 Americans. Of those jobs, nearly 120,000 are high-wage manufacturing jobs, which is double the percentage of manufacturing jobs compared to the private sector overall...Discussions about enabling more manufacturing in the United States are important, but let’s not forget that the United States already sustains a substantial manufacturing presence that is part of a larger global network. We cannot replace all global manufacturing with solely U.S. manufacturing without upsetting the entire biopharmaceutical supply chain to the detriment of patients...READ MORE
- As U.S. calls for stateside manufacturing, antibiotic maker Paratek gambles on ‘onshoring’ effort (fiercepharma.com)
...the U.S. government has shelled out big money for a stable and reliable supply of key drugs made on U.S. soil. For drugmakers accustomed to offshore manufacturing in cheaper countries, does it make any sense to onshore production to meet U.S. demand?...One company has a compelling argument, and it's taking big risks—and government funding—to test its hypothesis...Paratek Pharmaceuticals, maker of antibiotic Nuzyra, is kick-starting a three-year plan to build a government-funded, second supply chain in the U.S. in an effort to flesh out the nation's strategic supply of pandemic response drugs...The U.S. government, through its Biomedical Advanced Research and Development Authority, has dumped more than $300 million combined into helping Paratek build the U.S. supply line, which will stand apart from its current manufacturing network in Europe...READ MORE
- Merck, Lilly and Amgen win again in lawsuit over drug prices in TV ads. Will it stick? (fiercepharma.com)
The Trump administration's quest for drug prices in TV ads just took another hit—and it might be a fatal blow. A U.S. appeals court agreed with a lower court ruling that found the U.S. Department of Health and Human Services didn't have the authority to require them...It was a big win for Merck & Co., Eli Lilly and Amgen along with the Association of National Advertisers, which sued last June to block the rule that would have forced drugmakers to include list prices in TV ads...Their argument? HHS has no statutory authority to create the rule in the first place, and even if it did, the rule violates the First Amendment...READ MORE
- Show me the data: U.S. doctors skeptical of reported COVID breakthrough (reuters.com)
The report on...a powerful treatment for the new coronavirus brought skepticism along with optimism among U.S. doctors, who said the recent withdrawal of an influential COVID-19 study left them wanting to see more data...Global pressure to find a cure or vaccine has accelerated the process of reporting coronavirus study results, feeding confusion over whether therapies have been proven effective. One influential COVID study was withdrawn this month by respected British medical journal The Lancet over data concerns...Researchers in Britain said dexamethasone, used to fight inflammation in other diseases, reduced death rates of the most severely ill COVID-19 patients by around a third, and they would work to publish full details as soon as possible...But hours later South Korea’s top health official cautioned about the use of the drug for COVID-19 patients due to potential side effects...READ MORE
- ICYMI: Pharmacy benefit managers increasingly exclude medicines from formularies, restricting patient access (catalyst.phrma.org)
...CVS Caremark and Express Scripts, have once again increased the number of medicines on their standard formulary exclusion lists...As these PBMs control over half of the market combined, these changes have widespread implications for patients across the country...PBMs leverage their vast purchasing power and ability to exclude medicines from their standard formularies to negotiate large rebates and discounts from biopharmaceutical companies. PBMs then compel insurers and employers to use standard formularies by reducing the rebates offered to those who choose to adopt custom formularies without exclusions, which increases costs to the plan sponsor. In 2019, manufacturer rebates, discounts, fees and other price concessions grew to $175 billion. However, these rebates and discounts are typically not shared with patients at the point-of-sale...formulary exclusions can create additional barriers for patients who may experience challenges accessing prescribed treatments. These barriers to access can lead to health complications resulting from delayed treatment initiation or treatment disruption...READ MORE
- OHSU’s COVID-19 Study Accused Of Racial Bias (opb.org)
Charges of racial bias in the design of an Oregon study of COVID-19’s spread are raising questions about whether it will do anything to help Black and Latino communities, which have been among those hardest hit by the pandemic...“All it will be able to say is if white people are fine. And then we open up counties and people of color will die,” said Andres Lopez, research director for the Coalition of Communities of Color, a Portland-based alliance of organizations representing a number of different communities of color...The Key to Oregon Study, which plans to enlist 100,000 Oregonians and monitor them for a year for COVID-19 symptoms, will include what its designers are calling “a focus on enrolling people who fully represent the state, including our diversity in geography, socioeconomic status and communities of color.”...critics doubt Key to Oregon will succeed in its goal. They say the study design is fundamentally flawed, and that those flaws could have been avoided if people of color had been brought to the table when the study was being created...READ MORE
-Responding with listening sessions
-Advocates say study design suppresses Black and Latino voices
-OHSU methodology overlooks lessons of the past
-The principle of ‘nothing about us without us’
-OHSU researchers respond - Rising Costs Explain Why Canada Is Switching to Biosimilars (centerforbiosimilars.com)
Following in the footsteps of British Columbia, other Canadian provinces are working to implement their own biosimilars initiatives that would switch patients from some of the most costly reference biologics to biosimilar counterparts...A look at the numbers explains why Canadian provinces are introducing automatic biosimilar switching policies...In Ontario, one of 4 provinces that are moving forward with forced switching, a total of $800,000 was spent on publicly funded biologic medications in 2018, up nearly 3-fold from $259.4 million in 2010, and the projection is for the total to reach $1 billion in 2021...“The Biosimilars Initiative is a result of PharmaCare’s evidence-informed strategy to better optimize our public resources, get the best value for new treatments and services, and improve access to biologic medications for patients,” BC government officials said...READ MORE
- Flu shot makers gear up—and get creative—for a critical vaccination season (fiercepharma.com)
With the novel coronavirus continuing its global spread and a second wave threatening the United States later this year, experts worry an influx of influenza patients and COVID-19 patients will hit U.S. hospitals at the same time...pharma's working to ramp up not only for the increased demand but also for the logistical challenges of vaccinating millions of people during a pandemic...Manufacturers distributed about 170 million flu vaccine doses to the U.S. last year. This year, they're aiming to increase that by about 20 million...Vaccine makers are already producing their doses, with plans to start shipping later this summer...READ MORE