- Pharma’s reputation is holding strong during COVID-19—and Harris Poll has some reasons (fiercepharma.com)
Pharma’s reputation is holding strong these days, the latest Harris Poll survey shows. That’s good news again for the industry—but why the change?...The Harris Poll asked Americans that question. And, perhaps unsurprisingly, the COVID-19 crisis is the reason...Seventy percent told the market researcher that the industry’s overall response to the pandemic is the main reason for their more positive feelings. Efforts to develop a vaccine (58%), develop or find treatments (56%), create diagnostic tests (56%) and protect medical professionals by providing masks and protective gear (46%) also rated as key reasons perceptions have changed...READ MORE
- EMA urged to release full clinical trial data upon authorizing Covid-19 treatments (statnews.com)
Amid worldwide clamor for Covid-19 medicines and vaccines, the European Medicines Agency is being urged by several international clinical evidence experts...to publish all trial data on the same day any product is authorized for use against the novel coronavirus...In a letter to the European regulator, four country directors from the independent watchdog Cochrane and leaders from Germany’s Institute for Quality and Efficiency in Health Care argued that it is critical to promptly release clinical study reports to support further research and proper medical care. The reports are go-to documents that contain myriad details about the methods and results of a clinical trial...READ MORE
- CMS Lowers Medicare Insulin Copays (drugtopics.com)Out-of-Pocket Insulin Costs Remain Stable for the Privately Insured (ajmc.com)
The Centers for Medicare & Medicaid Services is reducing insulin copays for seniors who are eligible for Medicare via an executive order from President Donald Trump...Participating enhanced Medicare Part D plans in 2021 will provide a broad set of insulins at a maximum $35 copay for a month’s supply of each type of insulin, the White House said in a fact sheet on the new initiative...CMS estimates that beneficiaries could save $446, or 66%, a year for their insulins, the agency said in a press release...READ MORE
- Fears of coronavirus second wave prompt flu push at U.S. pharmacies, drugmakers (reuters.com)
U.S. pharmacy chains are preparing a big push for flu vaccinations when the season kicks off in October, hoping to curb tens of thousands of serious cases that could coincide with a second wave of coronavirus infections...CVS Health Corp...said it is working to ensure it has vaccine doses available for an anticipated surge in customers seeking shots to protect against seasonal influenza...Rival chain Rite Aid Corp has ordered 40 percent more vaccine doses to meet the expected demand. Walmart Inc and Walgreens Boots Alliance said they also are expecting more Americans to seek these shots...READ MORE
- A top adviser for Trump is calling for more drug manufacturing in Puerto Rico. (fiercepharma.com)
The COVID-19 pandemic has underscored key weaknesses in the biopharma industry’s complex global supply chain, and now a top adviser for the Trump administration is calling on Congress to push for more manufacturing in Puerto Rico...White House trade adviser Peter Navarro faulted a “broken system” that pushes manufacturing offshore. He called on Congress to use the next round of COVID-19 relief to incentivize manufacturing on the island territory...Previously, a tax provision allowed U.S. companies to avoid paying federal taxes on profits from operations in Puerto Rico, but Congress phased that out over time...Plus, utility costs are significantly higher there than in the U.S., which has also led to a reduction in manufacturing. Pharma companies themselves have shuttered a number of drug plants in Puerto Rico in recent years...READ MORE
- Delays getting records means crucial virus questions go unanswered (reviewjournal.com)
Nevada Gov. Steve Sisolak has touted open government as a crucial aspect to the state’s response to the COVID-19 outbreak. “You deserve transparency,” he proclaimed during an April 8 news conference, a statement reflecting the Nevada Public Records Act’s promise of open access to most government documents...But records vital to evaluating how Sisolak’s administration and state agencies have navigated the unprecedented emergency have proved difficult to obtain...Even a simple request for daily reports on hospital capacity made in early April, which would have spanned only a few pages at the time, was met with a response from a senior policy analyst in Sisolak’s office to wait “eight to 10 weeks” to receive the record...Among the requested documents that state agencies have delayed in producing or denied access to are the following:
■ Emergency management plans related to disease outbreaks or widespread health emergencies. Officials took more than 40 days to deny the request.
■ Documents tracking testing of prison inmates and staff for COVID-19. Denied by officials after 13 days.
■ Written communications among top prison officials about COVID-19 testing. Officials said they would respond “in the next 45 days.”
■ Records related to Nevada’s government stockpile of personal protective gear for medical workers. Officials said it will take “eight to ten weeks or longer” to compile the documents....READ MORE
- Vaccine experts say Moderna didn’t produce data critical to assessing Covid-19 vaccine (statnews.com)
Heavy hearts soared Monday with news that Moderna’s Covid-19 vaccine candidate — the frontrunner in the American market — seemed to be generating an immune response in Phase 1 trial subjects…But was there good reason for so much enthusiasm?...based on the information made available...there’s really no way to know how impressive — or not — the vaccine may be...While Moderna blitzed the media, it revealed very little information — and most of what it did disclose were words, not data...Even the figures the company did release don’t mean much on their own, because critical information — effectively the key to interpreting them — was withheld...Experts suggest we ought to take the early readout with a big grain of salt...READ MORE
- Canada’s patented drug regulator delaying implementation of new pricing guidelines (ipolitics.ca)Drug pricing regulation changes paving the way for pharmacare: health minister (ipolitics.ca)
Canada’s patented drug regulator is delaying the implementation of new pricing guidelines until 2021, to make “significant changes” in response to feedback it received during public consultations...Changes to the patented medicines regulations, which were originally announced last year, were supposed to go into force on July 1, 2020...The delay was applauded by federal opposition Conservatives, who argued that the regulatory changes set aggressive price ceilings for some new therapies — particularly for rare disorders — that would all but ensure they would not be brought to the Canadian market...“I’m relieved that the voices of patients have been heard and the government has decided to delay the changes. However, we still have a lot of uncertainty and I expect the government to use this additional time to better consult with patients,” Conservative MP and party health critic Matt Jeneroux said in a statement...We could see a drug shortage if these changes go ahead as planned in six months. Canada will no longer be a competitive marketplace and drug companies will be reluctant to bring their therapies here...READ MORE
- U.S. sends Brazil 2 million doses of hydroxychloroquine, drug touted by Trump (reuters.com)
The United States has supplied Brazil with 2 million doses of hydroxychloroquine for use against the coronavirus, the two governments said...despite medical warnings about risks associated with the anti-malaria drug...The White House released a joint announcement on the drug, whose use has been touted both by U.S. President Donald Trump and Brazilian President Jair Bolsonaro, just days after the World Health Organization suspended testing it in COVID-19 patients because of safety concerns...HCQ will be used as a prophylactic to help defend Brazil’s nurses, doctors and healthcare professionals against the virus. It will also be used as a therapeutic to treat Brazilians who become infected...READ MORE
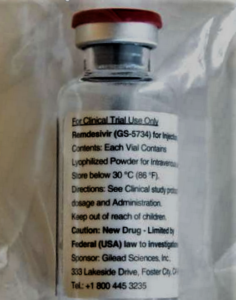
- Taxpayers paid to develop remdesivir but will have no say when Gilead sets price (chron.com)
One drug that has shown promise for treating COVID-19 is remdesivir, an experimental antiviral product...The drug that buoyed expectations for a coronavirus treatment and drew international attention for Gilead Sciences, remdesivir, started as a reject, an also-ran in the search for antiviral drugs. Its path to relevance did not begin until Robert Jordan cleared it…To make progress, Gilead needed help from U.S. taxpayers. Lots of help. Three federal health agencies were deeply involved in remdesivir's development every step of the way, providing tens of millions of dollars of government research support. Now that big government role has set up a political showdown over pricing and access...federal agencies have not asserted patent rights to Gilead's drug, potentially a blockbuster therapy worth billions of dollars. That means Gilead will have few constraints other than political pressure when it sets a price in coming weeks..."Without direct public investment and tax subsidies, this drug would apparently have remained in the scrap heap of unsuccessful drugs...READ MORE