- ISMP Awarded Contract from FDA to Conduct Medication Safety Self-Assessment (pharmacytimes.com)
The Institute for Safe Medication Practices has been awarded a contract from the FDA to assess medication safety practices in the perioperative setting from the time patients are prepared for a procedure until they are discharged home...Due to the complexity and fast-paced setting of perioperative care procedures, ISMP’s Medication Safety Self-Assessment for Perioperative Settings will provide facilities with a way to examine their medication safety practices, identify opportunities for improvement, and compare their experiences over time with the aggregate experience of demographically similar organizations...READ MORE
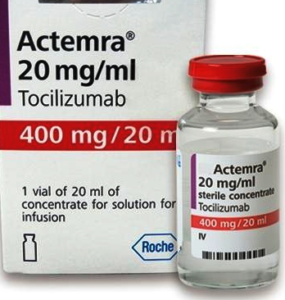
- China turns Roche arthritis drug Actemra against COVID-19 in new treatment guidelines (fiercepharma.com)
Some patients infected with the novel coronavirus can develop uncontrolled immune response, leading to potentially life-threatening damage to lung tissue. After seeing promising results in clinical practice, Chinese authorities now recommend a Roche arthritis drug to tackle that rampage...Roche’s blockbuster Actemra, first approved by the U.S. FDA in 2010 for rheumatoid arthritis, can now be used to treat serious coronavirus patients with lung damage, China’s National Health Commission said in its updated treatment guidelines for COVID-19...READ MORE
- What Pharmacies Are Doing to Mitigate Shortages Due to COVID-19 (drugtopics.com)
Chain pharmacies and US government agencies are doing everything they can to mitigate shortages of facemasks, hand sanitizers, and other products as consumers rush to purchase supplies due to coronavirus...fears..."The extreme demand in some areas certainly is affecting supplies," Chris Krese, spokesperson for NACDS, told Drug Topics®...Meanwhile, the FDA announced the first drug shortage due to COVID-19, but declined to list the drug in shortage...This information is considered proprietary...hand sanitizers and facemasks have been virtually stripped from store shelves, and that Amazon, Walmart, and other retailers’ web sites are running out of affordable hand sanitizers..."Due to high demand and to support all customers, we will be limiting the number of sanitization, cold and flu-related products to 5 each per order," Kroger said on its website...READ MORE
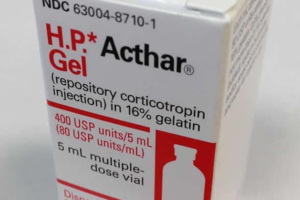
- Feds target Mallinckrodt after joining Medicaid rebate suit worth ‘hundreds of millions’ (fiercepharma.com)
Amid efforts to close a $1.6 billion opioid settlement, Mallinckrodt has fought tooth and nail to escape a massive Medicaid rebate bill for its controversial H.P. Acthar Gel. Instead of ceding ground, the U.S. government opted to take the fight right back to Mallinckrodt––and it could create even more trouble for the embattled drugmaker...The federal government has joined a False Claims Act whistleblower suit filed in Boston accusing Mallinckrodt of underpaying Medicaid rebates for Acthar by "hundreds of millions of dollars,"...READ MORE
- Pharmacy Week in Review: March 13 (pharmacytimes.com)
Nicole Grassano, PTNN, Pharmacy Week in Review, this weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- ‘Filthy’ equipment of suppository maker repaired with plastic wrap, FDA warning letter says (fiercepharma.com)
A suppository maker that has been a problem for the FDA in the past is at it again. An inspection of its New Jersey manufacturing facility found its equipment was “filthy” and some of it held together with tape and plastic wrap...On top of that, the warning letter for Acino Products, an over-the-counter maker of suppositories and topical solutions, said it could not come up with documents for microbiological testing...While the company pledged to make fixes and improvements, the FDA said it was not nearly enough...READ MORE
- This Week in Managed Care: March 6, 2020 (ajmc.com)
Christina Mattina, welcome to This Week in Managed Care from the Managed Markets News Network
- What we know about drugmakers’ response to the new coronavirus (biopharmadive.com)
The new coronavirus is moving quickly around the world. Since December, when it was first identified in Wuhan, China, the virus has spread to more than 100 countries. Some 105,000 cases have been confirmed, though the true number is surely higher, and at least 3,500 people have died. Already, the virus known as SARS-CoV-2 is straining healthcare systems in the hardest-hit regions, and threatens to do the same elsewhere as the infection total grows...BioPharma Dive compiled a roundup of our coronavirus coverage so far. There are many unanswered questions, and the drug industry's response is just beginning. We will update the roundup as we get more information...READ MORE
- Indian export group says European industry ‘panicking’ over APIs (in-pharmatechnologist.com)Indian government shuts down export of certain APIs due to coronavirus (in-pharmatechnologist.com)
After the Indian government moved to limit the export of APIs, an Indian export association says fear is spreading amongst European businesses over supply...The news broke...that the Indian government had decided that the export of 26 active pharmaceutical ingredients and formulations would be placed under restriction...As a result, Dinesh Dua, chairman of the Pharmaceuticals Export Promotion Council of India, told Reuters, that European industry was ‘panicking’...“I am getting a huge number of calls from Europe because it is very sizeably dependent on Indian formulations and we control almost 26% of the European formulations in the generic space.”...READ MORE
- March 6 Week in Review (pharmacytimes.com)
Nicole Grassano, PTNN, Pharmacy Week in Review, this weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.