- Merck CEO Frazier finds fault in new studies suggesting pharma profits are too high (fiercepharma.com)
Is the pharma industry hiking up drug prices to boost profitability at the expense of hardworking Americans? That question—a favorite of politicians on both sides of the aisle—is at the heart of a special issue of the Journal of the American Medical Association, which features a collection of articles that aim to provide a well-rounded answer...Perhaps the most damning of the studies compares the profits of the 35 biggest pharmaceutical companies to the S&P 500 as a whole. The study...found that between 2000 and 2018 the median annual gross profit margin of Big Pharma was 76.5%, versus just 37.4% for the S&P 500 companies. Earnings before taxes and net income margins were also significantly higher among pharma companies, the authors reported...Merck CEO Kenneth Frazier had his own set of worries about the new JAMA studies, though he was more focused on fairness—or lack thereof...READ MORE
- Coronavirus spurs India to restrict exports of 2 dozen drugs (fiercepharma.com)India's restrictions on API exports only temporary, official says: report (fiercepharma.com)
While eyes have been on China for signs that COVID-19 might result in drug shortages, India has come up with a surprise of its own. The country, which accounts for about 40% of U.S. generic drugs, has halted exports of more than two dozen APIs and drugs...India’s Directorate General of Foreign Trade today announced it was restricting 26 APIs and formulations until further notice...The government gave no further explanation, but Dinesh Dua, chairman of the Pharmaceuticals Export Promotion Council of India, told Reuters, “Irrespective of the ban, some of these molecules may face shortages for the next couple of months.” If interruptions from the virus get worse, he said, some shortages may become “acute.”...READ MORE
- Pharmacy Groups Express Concerns About Health Care Megamergers (drugtopics.com)
In new comments to the US government, several pharmacy groups say that mergers of major companies, such as CVS Health and Aetna, have had significant anticompetitive effects without improving the cost and quality of health care...The Alliance for Pharmacy Compounding, American Pharmacists Association, American Society of Consultant Pharmacists, National Alliance of State Pharmacy Associations, and National Community Pharmacists Association collectively urged for antitrust policy reform in a joint comment to the Federal Trade Commission and the Department of Justice Antitrust Division...READ MORE
- This Week in Managed Care: February 28, 2020 (ajmc.com)
Christina Mattina, welcome to This Week in Managed Care from the Managed Markets News Network
- CAR-T—the Future of Medical Progress Is Now (realclearhealth.com)
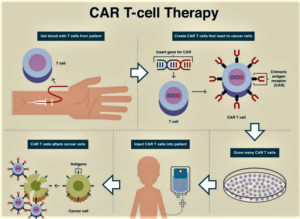
Personalized medicine is the future of medical progress...For instance, an immunotherapy treatment called chimeric antigen receptor T-cell therapy is accessible now, and the Trump administration has the opportunity to make it widely available to Medicare beneficiaries...over 70 Members of Congress—Democrats, Republicans, liberals, and conservatives—sent a letter to Seema Verma, administrator of the Centers for Medicare and Medicaid Services, commending the administration for “ensuring Medicare patients nationwide have access” to this life-saving treatment. The Congressional letter goes on to ask the administration to “ensure that hospitals are appropriately reimbursed so they may continue to provide” CAR-T therapy to America’s seniors...without appropriate reimbursement policy, a Medicare patient could be denied access to a treatment that would save his or her life. Without proper reimbursement by Medicare, providers simply will not be able to offer it as an option, especially in rural areas as patients must stay near a treatment center for four weeks to be monitored...READ MORE
- Cardinal Health pays SEC $8.8 million to settle China FCPA offenses (fcpablog.com)
Cardinal Health paid the SEC $8.8 million Friday to settle FCPA (Foreign Corrupt Practices Act) offenses related to a Chinese subsidiary that provided marketing services...In an internal administrative order, the SEC charged Cardinal Health with violating the FCPA’s books and records and internal accounting controls provisions...Dublin, Ohio-based Cardinal Health agreed to disgorged $5.4 million to the SEC, plus prejudgment interest of $916,887, and pay a civil penalty of $2.5 million...In one instance, a marketing account it administered for a UK- based pharmaceutical manufacturer was terminated in 2013 after Cardinal’s CEO received an internal report alleging that Cardinal China employees were using the account to “bribe employees of China’s Center for Disease Control.”...READ MORE
- Sandoz pledges ‘stable’ pricing for generics, antibiotics amid COVID-19 supply chain worries (fiercepharma.com)
COVID-19's global spread has raised fears over drug shortages and price spikes, but Novartis' Sandoz is taking an early step to allay those concerns. The drugmaker, a top player in generics and off-patent antibiotics, pledged “stable” pricing for certain essential drugs and antibiotics amid the crisis...No matter how the supply chain situation plays out, Sandoz pledged to “keep prices stable for certain essential medicines it markets commercially," CEO Richard Saynor said in a statement...The commitment comes amid fears that the global supply chain will face disruption from an outbreak that’s so far taken its biggest toll in China, a manufacturing powerhouse where many of the pharmaceutical industry's starting materials are made...READ MORE
- The Virus and the Supply Chain (city-journal.org)
The new coronavirus outbreak may be very bad for your health but not only for the reasons you imagined. This coronavirus is less likely to harm you directly than to injure you through its impact on your other medical needs...COVID-19 is more likely to harm Americans indirectly because the U.S. is increasingly reliant on drugs either directly sourced from China or made from intermediate chemicals...While 90 percent of the finished drugs Americans take are generics, most are manufactured overseas, primarily in India and China. Even India...relies on China for 80 percent of the APIs it uses in drug production...COVID-19 has resulted in massive disruption of Chinese manufacturing. It’s only a matter of time until this translates into supply disruptions for China-dependent customers...Coronavirus has created concerns about not only the quantity of Chinese medical products available but also about the virus’s effect on quality. China does not effectively regulate Chinese drug manufacturers. Multiple episodes have cast doubt on the safety and efficacy of their products...READ MORE
- Bristol-Myers Squibb reaches tentative deal to end long-running Medicaid rebate lawsuit (fiercepharma.com)
Lawsuits over Medicaid rebates are a fact of life in pharma, and in one particularly long-running case, Bristol-Myers Squibb stands accused of fiddling with its prices to lower payments. Now, after seven years in litigation, that lawsuit could be nearing an end...Bristol reached an "agreement in principle" in January to settle a 2013 whistleblower lawsuit accusing the company of manipulating the average manufacture price of its drugs in order to underpay Medicaid rebates between 2007 and 2016, according to an SEC filing...READ MORE
- FDA Issues Final Rule Expanding Availability of Insulin Products (fdanews.com)FDA Works to Ensure Smooth Regulatory Transition of Insulin and Other Biological Products (fda.gov)
The FDA issued a final rule amending the definition of “biological product” to include chemically synthesized polypeptides, a category of products that includes all insulins currently on the market...The final rule clears the way for the agency’s March 23 transition of filings for chemically synthesized polypeptides, including insulin products, from new drug applications to biologics license applications...The FDA anticipates that the change will add more choices for patients and potentially reduce insulin prices. The final rule “will open new pathways for manufacturers to bring biosimilar and interchangeable versions of insulin and other transitioning products to market, facilitating greater competition,” said FDA Commissioner Stephen Hahn.