- With new report, ICER puts itself at center of drug pricing storm (biopharmadive.com)
Pharmaceutical companies often preach value. Yet, for seven top-selling drugs, prices went up in 2017 and 2018 despite limited new evidence showing patients receiving treatment experienced greater benefit, according to a new report...Taken together, the price hikes added more than $5 billion to U.S. spending on those drugs over the two-year period, a study published...by the Institute for Clinical and Economic Review (ICER) said...The finding is the latest challenge from a group known for criticizing the drug industry's approach to pricing, and is likely to stir debate at a time when Congress is considering legislation to curb rising drug costs...READ MORE
- CVS drug coverage plan based on outside pricing review is off to a slow start (reuters.com)
A CVS Health Corp health plan that uses an outside drug pricing group to help it decide whether to cover certain new medicines has gained little traction with customers...has drawn fierce criticism from patient advocacy groups...The plan, launched a year ago, is based on analyses by the Institute for Clinical and Economic Review, a Boston-based group that assesses effectiveness of drugs to determine appropriate prices...Using ICER’s cost effectiveness assessment, CVS decides whether to include second or third medicines entering the market if there are already similar ones in the plan...Opposition to the CVS plan is part of much broader concerns cited by drug companies and advocacy groups, many of which receive funding from the pharmaceutical industry...More than 50 groups, including drugmakers, PhRMA, the industry’s main lobby group, and other advocacy groups, have provided comment during a public input period included in a review by ICER of its assessment methods. Many asked ICER to eliminate price recommendations from its efficacy analyses...READ MORE
- Nevada levies $17 million in fines on drug companies for noncompliance with diabetes drug transparency law (thenevadaindependent.com)
The state is imposing $17.4 million in fines on 21 diabetes drug manufacturers that have either failed to comply with or were many months late in complying with a drug pricing transparency law passed two years ago...The fines, which the state is allowed to assess at $5,000 a day, range from $735,000 for one company that submitted the required drug pricing data the same day it received a final notice from the state — but 147 days after the reporting deadline — to $910,000 for eight companies that still have yet to report the required information. The Department of Health and Human Services told the companies in letters sent this week that they have 30 days to either pay the fines in full or 10 days to request an informal dispute resolution meeting with the state...READ MORE
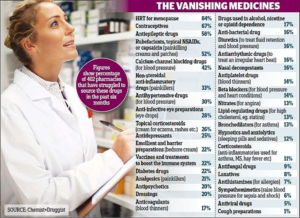
- Crisis at chemists: Pharmacies are ‘barely coping’ as dozens of popular medicines run short leaving hundreds of thousands of patients in the lurch (dailymail.co.uk)
Pharmacists have warned of shortages for every major type of medicine – including painkillers, contraceptives and diabetes pills...A poll of community pharmacists...revealed the dramatic extent of the drugs crisis that is hitting hundreds of thousands of patients in the UK...Pharmacists said they are living ‘on a knife-edge’ due to the mass shortages, which are caused by a perfect storm of manufacturing problems...READ MORE
- California bans pharma’s infamous ‘pay-for-delay’ deals (fiercepharma.com)
When generic challengers come for a branded med’s patent, drugmakers have in the past chosen to pony up and stall their rivals with an anticompetitive pact better known as “pay for delay.” In an effort to keep drug prices down, California is looking to end the practice...a new bill...will make California the first state to ban pay-for-delay deals in pharma...AB 824, will make it unlawful for companies to exchange anything of value in return for a halt to patent challenges from generic drugmakers. That new measure could open the door to a range of civil suits against companies seeking to keep generic competitors off the market...READ MORE
- This Week in Managed Care: October 4, 2019 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- 340B allies rally Congress to ensure Pelosi drug price plan doesn’t imperil discounts (fiercehealthcare.com)
A 340B advocacy group is imploring congressional allies to ensure House Speaker Nancy Pelosi’s drug prices plan won’t prevent hospitals from getting discounts under the program...The hospital industry-backed advocacy group 340B Health sent a letter to the House Energy & Commerce Committee...extolling the virtues of the program. The letter comes after the release of Pelosi’s Lower Drug Costs Act, which would call for the Department of Health and Human Services secretary to select at least 25 drugs a year to negotiate for a lower price...Currently, the legislative text says that for each year that a negotiated price is applied that drug shall “not be considered a covered outpatient drug subject to an agreement under … 340B.”...READ MORE
- After fanfare, Civica Rx delivers its 1st drugs (biopharmadive.com)
...Civica Rx has delivered on its promise to supply hospitals with generic medications...Intermountain Health and other nonprofit systems joined forces in hopes of providing more predictable pricing and access to drugs...The company pledges that every hospital, regardless of size, will have the same access to products, and says it will only set one market price. Larger systems will not receive discounts on larger volume purchases and every hospital will have the same contracting terms...READ MORE
- October 4 Pharmacy Week in Review (pharmacytimes.com)
Nicole Grassano, PTNN, Pharmacy Week in Review, this weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Google parent Alphabet hires former FDA head Robert Califf to lead health strategy and policy (fiercehealthcare.com)
Google parent company Alphabet has hired former U.S. Food and Drug Administration Commissioner Robert Califf, M.D., to...serve as head of strategy and policy across the company's Google Health and Verily Life Sciences enterprises beginning Nov. 18...He served at the FDA during President Barack Obama’s administration, first as deputy commissioner for medical products and tobacco from 2015 to 2016 then as commissioner of food and drugs from 2016 to 2017...READ MORE