- FDA will require 50% efficacy for COVID-19 vaccines. How high is that bar? (fiercepharma.com)Development and Licensure of Vaccines to Prevent COVID-19 Guidance for Industry (fda.gov)
Coronavirus vaccine developers now have some advice from the FDA: To win approval, any vaccine must be at least 50% effective in preventing the disease...FDA Commissioner Stephen Hahn plans to roll out that guidance...It sets a bar about on par with a flu shot's performance in a good year—but it falls short of some expert recommendations for arresting the virus' spread...The agency also won’t approve a shot based on its ability to create antibodies in patients’ blood...Despite the urgency of this particular vaccine hunt, the FDA “will not reduce its standards or cut corners in its review to approve a vaccine,”...READ MORE
- Vaccine experts say Moderna didn’t produce data critical to assessing Covid-19 vaccine (statnews.com)
Heavy hearts soared Monday with news that Moderna’s Covid-19 vaccine candidate — the frontrunner in the American market — seemed to be generating an immune response in Phase 1 trial subjects…But was there good reason for so much enthusiasm?...based on the information made available...there’s really no way to know how impressive — or not — the vaccine may be...While Moderna blitzed the media, it revealed very little information — and most of what it did disclose were words, not data...Even the figures the company did release don’t mean much on their own, because critical information — effectively the key to interpreting them — was withheld...Experts suggest we ought to take the early readout with a big grain of salt...READ MORE
- U.K. grants new vaccine manufacturing center £131M as researchers race to deliver a COVID-19 shot (fiercepharma.com)Vaccines Manufacturing and Innovation Centre to open 12 months ahead of schedule (gov.uk)
The COVID-19 pandemic has forced companies and organizations worldwide to change courses, and the U.K.’s Vaccines Manufacturing and Innovation Centre is no different. With a new £131 million contribution from the U.K. government, the center aims to both speed up and expand upon its prior ambitions...“This is a transformational moment for our organization to be part of a national and global response, and we’re very proud to be part of that,” Duchars said. Even beyond COVID-19, VMIC also aims to be a partner for vaccine developers worldwide that could tap its manufacturing and development expertise for various diseases...READ MORE
- Fair price for Gilead’s COVID-19 med remdesivir? $4,460, cost watchdog says (fiercepharma.com)
While Gilead has yet to present a marketing plan for the first coronavirus treatment to have shown clinical benefits in a well-designed randomized study, the Institute for Clinical and Economic Review (ICER)—which routinely weighs in on drug costs—says the drug is cost-effective at $4,460 per course of treatment...Even at $1,000 per patient, less than a quarter of ICER's fair price, Gilead could rake in $1 billion in sales this year...The company’s now bolstering supply with the aim to treat 1 million patients by the end of the year...For now, Gilead is donating remdesivir to the U.S. government for allocation, and it's pledged to continue giving doses away until its current supply chain is exhausted...Drugmakers aren’t obligated to follow ICER’s pricing limits, and they often find themselves at odds with each other...READ MORE
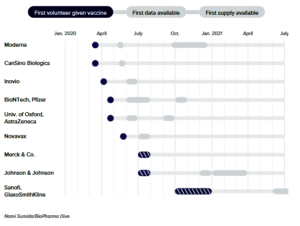
- The coronavirus vaccine frontrunners have emerged. Here’s where they stand
Fast progress by several companies has spurred hopes that a vaccine is coming soon, spurring jockeying among governments to secure supplies…Scientists, drugmakers and governments are moving with unprecedented speed to deliver a vaccine to protect against the new coronavirus…The fastest of them have already delivered preliminary data from human studies, and further results from others should come quickly as the year progresses…The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world…Vaccine frontrunners plan for fast development…READ MORE
- Taxpayers paid to develop remdesivir but will have no say when Gilead sets price (chron.com)
One drug that has shown promise for treating COVID-19 is remdesivir, an experimental antiviral product...The drug that buoyed expectations for a coronavirus treatment and drew international attention for Gilead Sciences, remdesivir, started as a reject, an also-ran in the search for antiviral drugs. Its path to relevance did not begin until Robert Jordan cleared it…To make progress, Gilead needed help from U.S. taxpayers. Lots of help. Three federal health agencies were deeply involved in remdesivir's development every step of the way, providing tens of millions of dollars of government research support. Now that big government role has set up a political showdown over pricing and access...federal agencies have not asserted patent rights to Gilead's drug, potentially a blockbuster therapy worth billions of dollars. That means Gilead will have few constraints other than political pressure when it sets a price in coming weeks..."Without direct public investment and tax subsidies, this drug would apparently have remained in the scrap heap of unsuccessful drugs...READ MORE
- Moderna Reports Positive Early Data for COVID-19 Vaccine Candidate (drugtopics.com)Moderna taps $1.34B stock offering to bankroll its promising COVID-19 vaccine (fiercepharma.com)
Moderna announced new interim clinical data results for its coronavirus disease 2019 (COVID-19) vaccine candidate from a phase 1 study...The investigational vaccine, called mRNA-1273, was launched...on March 16, making it the first trial to be started in humans for a vaccine for this virus...Tal Zaks, MD, PhD, chief medical officer at Moderna, said in a statement. “When combined with the success in preventing viral replication in the lungs of a pre-clinical challenge model at a dose that elicited similar levels of neutralizing antibodies, these data substantiate our belief that mRNA-1273 has the potential to prevent COVID-19 disease and advance our ability to select a dose for pivotal trials.”...Moderna expects to begin its phase 3 study in July 2020...READ MORE
- EMA urged to release full clinical trial data upon authorizing Covid-19 treatments (statnews.com)
Amid worldwide clamor for Covid-19 medicines and vaccines, the European Medicines Agency is being urged by several international clinical evidence experts...to publish all trial data on the same day any product is authorized for use against the novel coronavirus...In a letter to the European regulator, four country directors from the independent watchdog Cochrane and leaders from Germany’s Institute for Quality and Efficiency in Health Care argued that it is critical to promptly release clinical study reports to support further research and proper medical care. The reports are go-to documents that contain myriad details about the methods and results of a clinical trial...READ MORE
- Tracking biopharma’s response to the new coronavirus (biopharmadive.com)
The new coronavirus moved around the world with lightning speed. Since December, when it was first identified in Wuhan, China, nearly every country has reported cases of infection. More than 4.9 million cases have been confirmed...and over 327,000 people have died...Dozens of drugmakers have started work on vaccines to protect against the virus or medicines to treat COVID-19...Hundreds of studies are underway in search of an effective treatment, testing mostly repurposed HIV or influenza drugs...For the biopharma industry, the virus has disrupted business on a broad scale. Many companies source chemicals or pharmaceutical ingredients from factories across the globe, creating supply chain challenges...The epidemic's impact on clinical trials was significant, causing numerous delays to enrollment or postponements to studies of treatments for other diseases...BioPharma Dive compiled a roundup of our coronavirus coverage so far. There are many unanswered questions, and the drug industry's response is only in its opening stages...READ MORE
- Covid-19 pandemic: investigating the treatment landscape with IFPMA (pharmaceutical-technology.com)Investigational Drugs in the Pipeline for COVID-19 (drugtopics.com)
It is going to take at least a year for a Covid-19 vaccine to be available; in the meantime, drugs, both novel and repurposed, could provide a quicker alternative for the pharma industry to help resolve the ongoing novel coronavirus pandemic...the pharma industry has focused its efforts on leveraging its disease tackling expertise to find medical solutions to this novel viral disease...“A lot of the focus over the past few weeks has been on vaccines,” noted International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) director-general Thomas Cueni...it is highly unlikely a vaccine will be available for at least 12 to 18 months, drug studies are dominating the short-term response to this public health crisis...there are over 130 treatments in the pipeline for Covid-19 – 77 of which are repurposed and 68 are novel – which is “really good news for all of us”. These range from antimalarials and antivirals to anti-inflammatory drugs and plasma-based treatments...READ MORE