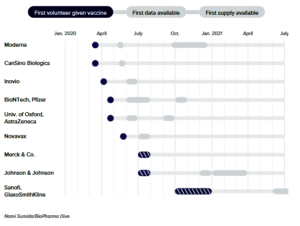
- The coronavirus vaccine frontrunners have emerged. Here’s where they stand
Fast progress by several companies has spurred hopes that a vaccine is coming soon, spurring jockeying among governments to secure supplies…Scientists, drugmakers and governments are moving with unprecedented speed to deliver a vaccine to protect against the new coronavirus…The fastest of them have already delivered preliminary data from human studies, and further results from others should come quickly as the year progresses…The goal, at least in the U.S., is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world…Vaccine frontrunners plan for fast development…READ MORE
- Tracking biopharma’s response to the new coronavirus (biopharmadive.com)
The new coronavirus moved around the world with lightning speed. Since December, when it was first identified in Wuhan, China, nearly every country has reported cases of infection. More than 4.9 million cases have been confirmed...and over 327,000 people have died...Dozens of drugmakers have started work on vaccines to protect against the virus or medicines to treat COVID-19...Hundreds of studies are underway in search of an effective treatment, testing mostly repurposed HIV or influenza drugs...For the biopharma industry, the virus has disrupted business on a broad scale. Many companies source chemicals or pharmaceutical ingredients from factories across the globe, creating supply chain challenges...The epidemic's impact on clinical trials was significant, causing numerous delays to enrollment or postponements to studies of treatments for other diseases...BioPharma Dive compiled a roundup of our coronavirus coverage so far. There are many unanswered questions, and the drug industry's response is only in its opening stages...READ MORE
- Expanding The Tent: Improving Trial Participation Among Under-Represented Patient Populations (invivo.pharmaintelligence.informa.com)
The biopharma industry has struggled to recruit patients into clinical trials that adequately reflect the diverse patient populations they hope to reach with new products. Failure to improve minority subgroup participation now will cost trial sponsors later...New Research from the Tufts Center for the Study of Drug Development reveals the extent to which minority groups are absent from clinical trials supporting new drug and biologic approvals...Additional tools are emerging to help sponsors effectively recruit and enroll underrepresented patient populations in clinical trials...READ MORE
- US begins first study of coronavirus treatment, testing Gilead’s remdesivir (flickr.com)Gilead commits to in-house development of coronavirus treatment hopeful (biopharmadive.com)Hong Kong plans $15 billion spending to support its economy amid coronavirus outbreak (cnbc.com)Coronavirus (nytimes.com)Coronavirus COVID-19 Global Cases by Johns Hopkins CSSE (gisanddata.maps.arcgis.com)
Two clinical trials testing potential treatments for the new coronavirus spreading from China are getting underway in Nebraska and Washington, marking a step forward in the U.S. efforts to find a therapy or vaccine for the pneumonia-like illness caused by the virus...The trial, which is designed to expand to include new centers and experimental drugs over time, will test first remdesivir, an antiviral originally developed by Gilead for use against the Ebola virus. Experts view the drug as among the more promising existing therapies for potential use against the new coronavirus, now called SARS-CoV-2...READ MORE
- OHSU’s COVID-19 Study Accused Of Racial Bias (opb.org)
Charges of racial bias in the design of an Oregon study of COVID-19’s spread are raising questions about whether it will do anything to help Black and Latino communities, which have been among those hardest hit by the pandemic...“All it will be able to say is if white people are fine. And then we open up counties and people of color will die,” said Andres Lopez, research director for the Coalition of Communities of Color, a Portland-based alliance of organizations representing a number of different communities of color...The Key to Oregon Study, which plans to enlist 100,000 Oregonians and monitor them for a year for COVID-19 symptoms, will include what its designers are calling “a focus on enrolling people who fully represent the state, including our diversity in geography, socioeconomic status and communities of color.”...critics doubt Key to Oregon will succeed in its goal. They say the study design is fundamentally flawed, and that those flaws could have been avoided if people of color had been brought to the table when the study was being created...READ MORE
-Responding with listening sessions
-Advocates say study design suppresses Black and Latino voices
-OHSU methodology overlooks lessons of the past
-The principle of ‘nothing about us without us’
-OHSU researchers respond - U.K. grants new vaccine manufacturing center £131M as researchers race to deliver a COVID-19 shot (fiercepharma.com)Vaccines Manufacturing and Innovation Centre to open 12 months ahead of schedule (gov.uk)
The COVID-19 pandemic has forced companies and organizations worldwide to change courses, and the U.K.’s Vaccines Manufacturing and Innovation Centre is no different. With a new £131 million contribution from the U.K. government, the center aims to both speed up and expand upon its prior ambitions...“This is a transformational moment for our organization to be part of a national and global response, and we’re very proud to be part of that,” Duchars said. Even beyond COVID-19, VMIC also aims to be a partner for vaccine developers worldwide that could tap its manufacturing and development expertise for various diseases...READ MORE
- ‘Directing’ evolution to identify potential drugs earlier in discovery (sciencedaily.com)
Scientists have developed a technique that could significantly reduce the time of discovering potential new antibody-based drugs to treat disease...New research...has resulted in a technique that allows fragments of antibodies to be screened for susceptibility to aggregation caused by structure disruption much earlier in the drug discovery process...a significant problem has been the failure rate of candidates upon manufacturing at industrial scale. This often only emerges at a very late stage in the development process -- these drugs are failing at the last hurdle...READ MORE
How the target proteins are screened
Directed evolution
- Vaccine experts say Moderna didn’t produce data critical to assessing Covid-19 vaccine (statnews.com)
Heavy hearts soared Monday with news that Moderna’s Covid-19 vaccine candidate — the frontrunner in the American market — seemed to be generating an immune response in Phase 1 trial subjects…But was there good reason for so much enthusiasm?...based on the information made available...there’s really no way to know how impressive — or not — the vaccine may be...While Moderna blitzed the media, it revealed very little information — and most of what it did disclose were words, not data...Even the figures the company did release don’t mean much on their own, because critical information — effectively the key to interpreting them — was withheld...Experts suggest we ought to take the early readout with a big grain of salt...READ MORE
- Moderna Reports Positive Early Data for COVID-19 Vaccine Candidate (drugtopics.com)Moderna taps $1.34B stock offering to bankroll its promising COVID-19 vaccine (fiercepharma.com)
Moderna announced new interim clinical data results for its coronavirus disease 2019 (COVID-19) vaccine candidate from a phase 1 study...The investigational vaccine, called mRNA-1273, was launched...on March 16, making it the first trial to be started in humans for a vaccine for this virus...Tal Zaks, MD, PhD, chief medical officer at Moderna, said in a statement. “When combined with the success in preventing viral replication in the lungs of a pre-clinical challenge model at a dose that elicited similar levels of neutralizing antibodies, these data substantiate our belief that mRNA-1273 has the potential to prevent COVID-19 disease and advance our ability to select a dose for pivotal trials.”...Moderna expects to begin its phase 3 study in July 2020...READ MORE
- Two generic drugs being tested in U.S. in race to find coronavirus treatments (reuters.com)
U.S. researchers, following the lead of scientists in other countries, have launched studies to see whether widely-available, low-cost generic drugs can be used to help treat the illness caused by the new coronavirus...But a 1,500-person trial, led by the University of Minnesota, began this week to see whether malaria treatment hydroxychloroquine can prevent or reduce the severity of COVID-19. Two other trials are studying the blood pressure drug losartan as a possible treatment for the disease...READ MORE