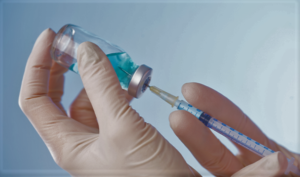
- Washoe County received doses from Moderna vaccine lot in question in California, though no allergic reactions reported (thenevadaindependent.com)
Washoe County Health District received doses from the same lot of Moderna’s coronavirus vaccine that California health officials have recommended providers stop administering after several people in the state experienced apparent severe allergic reactions...The health district administered doses from the same lot Moderna’s COVID-19 vaccine, 041L20A, to about 770 people at its drive-thru point of dispensing vaccination site...None of those individuals have reported severe allergic reactions, and there were no issues reported from patients who used the 15- and 30-minute waiting lots, which are set up to ensure that patients don’t experience any allergic reactions before driving off, after receiving the vaccine...READ MORE
- Vaccine Rollout Even Worse than Expected (biopharminternational.com)Operation Warp Speed Announces Changes to COVID-19 Vaccine Distribution Strategy (drugtopics.com)
Although there was much analysis and anticipation over the past year about the difficulties and challenges in distributing millions of doses of new vaccines across the nation, the process so far has been much less effective than anticipated...30 million doses have been distributed, but only 4 million people were vaccinated in December 2020, and just 13 million have received shots so far...A main problem was the administration’s delay in authorizing the Centers for Disease Control and Prevention to provide states with clear guidelines for updating existing local procedures for distributing vaccines that critical “last mile” to clinics and health centers... Uncertainty about vaccine shipments and supplies, at a time when hospitals were dealing with surges in COVID-19 infections and deaths, prompted medical centers to cancel in-house vaccination activities. And there still is confusion about whether clinics should hold back supplies for second doses or administer all on hand...READ MORE
- Trump administration accelerating launch of COVID-19 vaccinations in pharmacies (pharmacist.com)
In order to deliver COVID-19 vaccine to more people more quickly, the Trump administration intends to expedite rollout of its immunization partnership with pharmacies. The logic behind the concept is that getting vaccinated at a local pharmacy will be more accessible and efficient for many Americans than going to a hospital...READ MORE
- Becton Dickinson, ramping up syringe capacity, set to turn out 1B COVID-19 vaccine devices by year-end (fiercepharma.com)
With more than 1 billion BD injectors called for in the global fight against COVID-19, the company is looking at ways to support vaccination efforts up close and preparing for the future delivery of pandemic prophylactics...Becton Dickinson...last month surpassed 1 billion pandemic injection device orders globally...The company recently stumped up $1.2 billion to expand its pre-filled syringe capacity, sketching plans for a new plant in Europe, plus upgrades to six of the company’s existing sites in the U.S., Mexico, Europe and Japan. Those moves are expected to boost capacity by more than 50%...The company also teamed up with BARDA on a $70 million project to expand operations and dial up capacity for traditional needle and syringe devices at its Nebraska facilities. That upgrade, set to wrap in summer 2021, will provide the feds with priority access to “hundreds of millions” of injectors for COVID-19 vaccination efforts, plus supplies for future pandemics...READ MORE
- Biden’s COVID-19 challenge: 100M vaccinations in the first 100 days. It won’t be easy (fiercehealthcare.com)
...during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office...“This team will help get …at least 100 million COVID vaccine shots into the arms of the American people in the first 100 days,” Biden said...When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses...Either way, Biden may run into difficulty meeting that 100 million mark...READ MORE
- Biden aims to release all available COVID-19 shots in departure from Trump strategy (fiercepharma.com)
President Donald Trump's Operation Warp Speed promised 20 million COVID-19 vaccines administered to Americans by the end of 2020. Halfway into January, the rollout is still lagging behind those expectations...But President-elect Joe Biden's team is planning to shake up how doses are delivered after he takes office next week. Rather than holding back supply to ensure people can get their second doses, the Biden administration plans to release all available doses...It’s a departure from the Trump team’s approach at a time when the coronavirus vaccine rollout is failing to meet the pace needed to stem the pandemic...READ MORE
- Look out, pharma. A ‘tidal wave’ of side effect reports is coming amid COVID-19 vaccine rollouts (fiercepharma.com)
With COVID-19 vaccine launches gaining steam—and an unprecedented level of media coverage zeroed in—pharma companies of all stripes should brace not only for a wave of adverse event reports, experts say, but for lawsuits that could follow...With tens of millions of Americans set to be vaccinated, including many people at high risk of severe COVID-19, it's not just vaccine makers who need to actively look out for potential adverse events or drug interactions, lawyers with Sidley Austin said...All pharma companies—not just those involved in COVID-19 vaccine deliveries—can expect “a significant increase in volume of reports over the coming months,” Torrey Cope, a partner in the firm's Food, Drug and Medical Device Regulatory practice, said in an interview...READ MORE
- FDA Publishes COVID-19 Pandemic Recovery and Preparedness Plan Summary Report (biopharminternational.com)FDA COVID-19 Pandemic Recovery and Preparedness Plan (PREPP) Initiative: Summary ReportJanuary 2021 (fda.gov)
A report on the FDA PREPP initiative’s work in 2020 aims to strengthen the Agency’s response to future public health emergencies...FDA announced on Jan. 13, 2021 that it had published a report summarizing the work to date of the COVID-19 Pandemic Recovery and Preparedness Plan (PREPP) initiative, which was launched in August 2020 to strengthen the Agency’s response to the current pandemic and to future public health emergencies...FDA asked an independent, non-government organization to review its pandemic response, record its accomplishments and activities, and pinpoint future opportunities for consideration, an FDA press release said. The organization interviewed FDA leaders, organized listening sessions with external stakeholder groups, and partnered with subject matter experts on the FDA staff to generate a summary report...READ MORE
- China to provide COVID-19 vaccines free of charge: government official (reuters.com)
China will provide COVID-19 vaccines free of charge once they become available to general public, government authorities said...an official with China’s National Health Commission, told reporters that while manufacturing and transport of vaccines does have costs, the government will still be able to provide vaccines for free to individuals...“Our people don’t have to pay a single cent for the vaccine,”...READ MORE
- Not so fast: FDA warns of ‘premature’ changes to COVID-19 vaccine dosing in clash with Slaoui (fiercepharma.com)
The FDA warns against changing the dosing levels and schedules of COVID-19 vaccines by Moderna and Pfizer-BioNTech...Amid concerns over limited COVID-19 vaccine supplies, some have proposed tweaking the shots’ dosing to immunize more people. One suggestion came from none other than U.S. vaccine czar Moncef Slaoui, Ph.D...Any changes to currently authorized vaccine dosing regimens pose a “significant risk of placing public health at risk” and undermine “the historic vaccination efforts to protect the population from COVID-19,” FDA Commissioner Stephen Hahn, M.D., and Peter Marks, M.D., Ph.D., head of the agency’s biologics department, said in a statement...READ MORE