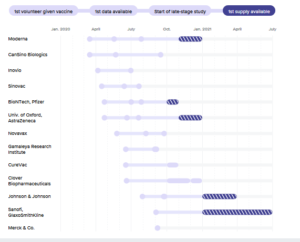
- The coronavirus vaccine frontrunners are advancing quickly. Here’s where they stand. (biopharmadive.com)
Fast progress has spurred hopes that several vaccines could succeed, spurring jockeying among governments to secure supplies...Scientists, drugmakers and governments are moving with unprecedented haste to develop a vaccine against the new coronavirus...The fastest of them have already delivered promising data from initial human studies, and further results from larger tests should come quickly over the next three to six months...The goal...is to have a vaccine ready for use in some fashion by the end of the year, or early next. Doing so would be a scientific feat with few parallels. No vaccine has ever been developed so quickly, never mind manufactured for the world...READ MORE
- Deregulate Pharmacists Now To Increase COVID-19 Vaccine Uptake (reason.com)
Enabling tens of millions of Americans to get themselves speedily vaccinated against COVID-19 will be a huge logistics challenge. A new policy brief from the Mercatus Center, a think tank at George Mason University, argues that we could greatly accelerate the process by removing the complex state regulations that prevent pharmacists from administering vaccines...The...report recommends that state regulators relax age restrictions on pharmacist-administered vaccinations; issue statewide standing orders authorizing pharmacists to administer vaccines without requiring a physician-written prescription for each patient; and revise regulations, as Oregon has, to permit pharmacists to administer all of the vaccines recommended by the CDC's Advisory Committee on Immunization Practices. The latter recommendation "prevents lag in vaccine administration due to boards or legislatures having to approve individually named vaccines for pharmacist administration."...READ MORE
- 5 questions ahead of next week’s FDA meeting on coronavirus vaccines (biopharmadive.com)Why this week’s meeting of an FDA advisory panel on Covid-19 vaccines matters (statnews.com)Vaccines and Related Biological Products Advisory Committee - 10/22/2020 (youtube.com)
Food and Drug Administration advisory committee meetings tend to be staid, albeit important, events typically watched by a few dozen company representatives, scientists, investors and patient advocates...But next week's hearing on coronavirus vaccine studies will be set against the backdrop of a presidential election 12 days later that could turn on the White House's response to the pandemic, likely making it a closely-watched affair of historic proportions...In the meantime, here are five important questions that could feature at next week's meeting...READ MORE
- How will vaccine safety be monitored following an emergency clearance? After a full approval?
- How long will immunity last and how would it be measured?
- How will vaccine-induced enhanced respiratory disease be monitored?
- Has enough data been collected on vulnerable groups?
- Will future vaccine trials need to measure COVID-19 cases, or will immune response data be enough?
- A vaccine alone won’t stop Covid-19. We also need a trusted plan for it (statnews.com)
Safe and effective vaccines represent the most effective way to restore the health and economic security disrupted by the Covid-19 pandemic. To help achieve that goal, the U.S. government launched Operation Warp Speed...to accelerate development and manufacturing of several Covid-19 vaccines, with a goal of having 300 million doses available to the U.S. population by January 2021...Operation Warp Speed is expediting vaccine development primarily by moving clinical trials forward without pauses between phases, and by scaling up manufacturing capacity before knowing if a candidate works...Covid-19 vaccines can help stop the pandemic only if people trust them and want to be vaccinated. To earn and keep the trust of the American people, our government needs to ensure three key needs are met before launching any immunization campaign...READ MORE
- Ensure transparency and confidence in FDA decisions
- Ensure robust active safety monitoring as Covid-19 vaccines are rolled out
- Ensure the distribution and administration of Covid-19 vaccines are equitable and well-executed
- Nursing homes to get free COVID-19 vaccines under Trump plan with CVS, Walgreens (usatoday.com)CVS, Walgreens make deal with Trump admin to quickly distribute COVID-19 vaccines to nursing homes (fiercehealthcare.com)
Americans living or working in long-term care facilities, including nursing homes and assisted care living centers, will receive COVID-19 vaccinations for free if and when they become available, the Trump administration said...The administration announced a partnership with the nation's two largest drug store chains, CVS and Walgreens, "to provide and administer" the vaccines with "no out-of-pocket costs" for the recipients...READ MORE
- AstraZeneca Covid-19 vaccine study put on hold due to suspected adverse reaction in participant in the U.K. (statnews.com)
A large, Phase 3 study testing a Covid-19 vaccine being developed by AstraZeneca and the University of Oxford at dozens of sites across the U.S. has been put on hold due to a suspected serious adverse reaction in a participant in the United Kingdom...A spokesperson for AstraZeneca, a front-runner in the race for a Covid-19 vaccine, said in a statement that the company’s “standard review process triggered a pause to vaccination to allow review of safety data.”...READ MORE