- Aspen Dental at Walgreens bring dental services to 2 Florida stores (drugstorenews.com)
Walgreens has undertaken a partnership to bring Aspen Dental-branded dental offices to two of its locations in Florida. The companies said the collaboration is aimed at helping transform Walgreens’ stores into health destinations that improve healthcare access in convenient settings...The companies held a “floss-cutting” ceremony at a location in Palatka, Fla. On Dec. 13, with the second Aspen Dental at Walgreens location set to open in the second quarter of 2019. The Aspen Dental office will offer free new patient exams and X-rays for patients without insurance and will feature an onsite denture lab to quickly turnaround custom dentures, repairs, relines or adjustments...
- FDA plan would ease regulations for prescription drug apps (biopharmadive.com)
The Food and Drug Administration is seeking public comment on a proposed framework for regulating software applications developed by drugmakers for use in conjunction with their prescription drug products...The new approach would treat most prescription drug apps, including dose calculators, symptom trackers and medication reminders, as promotional labeling...drugmakers would need only to submit to the agency copies of the content of what the apps display to consumers, following existing reporting requirements for promotional materials...In other cases, such as when a drugmaker wants to show that software has an effect on a clinical outcome and wants to include information about the software in the FDA-required drug labeling, prior FDA approval would be required...
- Advancing Toward the Goal of Global Approval for Generic Drugs: FDA Proposes Critical First Steps to Harmonize the Global Scientific and Technical Standards for Generic Drugs (fda.gov)
...FDA launched a Drug Competition Action Plan that focuses on three key areas designed to facilitate more generic competition, promote patient access, and improve the economics of developing generic medicines...While we’ve made substantial progress in fostering more competition by resolving obstacles that can make it difficult to win approval of generic versions of certain complex drugs, increasing the speed of generic approvals, and closing down ways that branded companies game the system to prolong drug monopolies, there’s still more work to be done...So we’re opening up some new policy fronts when it comes to our Drug Competition Action Plan. And we’re re-launching that plan for 2019 with some additional initiatives. Chief among them is a new effort that FDA has proposed to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), a key international body comprised of other regulatory authorities and the pharmaceutical industry: The pursuit of common global development standards for generic drugs...The ultimate goal of this global harmonization of scientific and technical requirements would be the attainment of a single global generic drug development program that can support simultaneous regulatory filings across multiple markets. Harmonization of these requirements is foundational to achieving a future goal of enabling global approval for high quality generic drugs.
- A new way to manufacture small batches of biopharmaceuticals on demand (news.mit.edu)
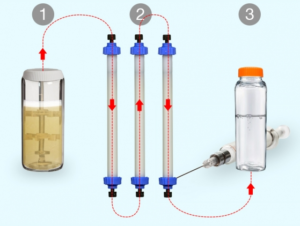
Biopharmaceuticals, a class of drugs comprising proteins such as antibodies and hormones, represent a fast-growing sector of the pharmaceutical industry. They’re increasingly important for “precision medicine” — drugs tailored toward the genetic or molecular profiles of particular groups of patients...Such drugs are normally manufactured at large facilities dedicated to a single product, using processes that are difficult to reconfigure. This rigidity means that manufacturers tend to focus on drugs needed by many patients, while drugs that could help smaller populations of patients may not be made...To help make more of these drugs available, MIT researchers have developed a new way to rapidly manufacture biopharmaceuticals on demand. Their system can be easily reconfigured to produce different drugs, enabling flexible switching between products as they are needed...Traditional biomanufacturing relies on unique processes for each new molecule that is produced...We’ve demonstrated a single hardware configuration that can produce different recombinant proteins in a fully automated, hands-free manner...
- Can blockchain stem the tide of counterfeit drugs in India? (pharmaceutical-technology.com)Blockchain use case: Healthcare supply chain (healthcareitnews.com)
The manufacture and distribution of fake prescription drugs is a global problem. Counterfeit products are causing a lot of damage to economies, industries, consumer health and safety. From the perspective of companies, they know they will suffer revenue and reputational losses whilst also incurring extra expenses for regulations, brand protection and legal advice if a solution to counterfeit drugs is not found...Now, an international partnership is bringing together IT giant Oracle, policy institute NITI Aayog, Apollo Hospitals and pharma manufacturer Strides Pharma Sciences to pilot a pioneering drug supply chain using blockchain digital ledger technology to try and tackle the issue...How can blockchain help companies to counter counterfeiters?...can this digital approach succeed where other anti-counterfeiting measures have failed?
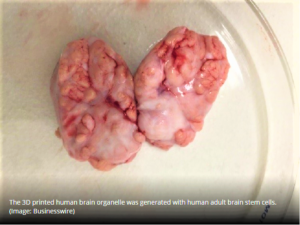
- 3D printed brain helps characterize 11 lead compounds, neuropathological research (outsourcing-pharma.com)
The stem cell research and therapeutics company Celprogen has identified and characterized 11 lead compounds for potential drug candidates for the treatment of Alzheimer’s, Parkinson’s, and Glioblastoma using its 3D model system...The 3D model system is unique since it represents the data generated closer to in-vivo data, eliminates animal studies, and represents patient’s brain physiological format...poor animal study design and reporting have raised concerns about whether current processes are the best way to conduct effective and efficient drug development...3D model systems aim to better inform risk-based decisions in drug development...
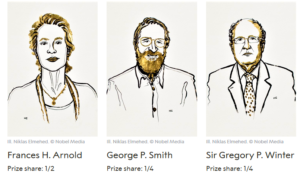
- Use of Evolution to Design Molecules Nets Nobel Prize in Chemistry for 3 Scientists (nytimes.com)The Nobel Prize in Chemistry 2018 (nobelprize.org)
Three scientists shared this year’s Nobel Prize in Chemistry for tapping the power of evolutionary biology to design molecules with a range of practical uses...Those include new drugs, more efficient and less toxic reactions in the manufacture of chemicals and plant-derived fuels to replace oil, gas and coal extracted from the ground...Half of the prize and the accompanying $1 million...Stockholm, went to Frances H. Arnold, a professor of chemical engineering at the California Institute of Technology. She is only the fifth woman to win a chemistry Nobel and the first since 2009...The other half of the prize is shared by George P. Smith, an emeritus professor of biological sciences at the University of Missouri, and Gregory P. Winter, a biochemist at the M.R.C. Laboratory of Molecular Biology in England...The prize highlights the narrowing of the gap between biology and some fields of chemistry as chemists turn to nature for inspiration...
- Walgreens, FedEx partner on next-day Rx delivery nationwide (drugstorenews.com)
Walgreens and FedEx are teaming up...The companies have launched Walgreens Express, a service that will provide next-day delivery nationwide, as well as same-day delivery in select markets...Patients who are enrolled in Walgreens text alerts will get a text message notification when their qualifying prescriptions are ready. They can then follow a process to choose to have their prescription delivered the next day for a $4.99 fee...Walgreens said most prescription orders are available to be delivered by the next business day, it said some prescription benefit plans and insurance plans do not allow home delivery, encouraging patients to talk to a pharmacist with any questions.
- FDA unveils open source code for collecting patient data (healthcareitnews.com)FDA’s MyStudies Application (App) (fda.gov)
The Food and Drug Administration launched a new app Wednesday to gather data for clinical trials and other research directly from patients...The FDA released the MyStudies app source code to the public, allowing developers and researchers to tailor the app to suit their research needs. The agency designed the app to facilitate the use of real-world data in research...Patients can submit real-world data to the app via their mobile devices. Researchers can then link those data to other electronic health information. The goal is to improve drug development...“Better capture of real world data, collected from a variety of sources, has the potential to make our new drug development process more efficient, improve safety and help lower the cost of product development,” FDA Commissioner Dr. Scott Gottlieb said in a statement. “Our hope is that the collection of more real world data directly from patients, using a secure app, will lead to more efficient product development and assist with safety monitoring.”
- Impatient patients turn to online ‘buyers club’ for new drugs (reuters.com)
Frustrated by delays in new medicines reaching their own country, a small but growing number of patients are turning to an online broker that bills itself as a legal version of the Dallas Buyers Club...While regulators warn of the risk of buying drugs online, the Amsterdam-based Social Medwork sees its network of trusted suppliers as filling a gap in the market for the latest drugs against diseases such as cancer, migraine and multiple sclerosis...Now it is looking to raise its profile and expand, by signing up former EU Commissioner Neelie Kroes to its supervisory board and securing 1.5 million euros ($1.73 million) in new funding from the Social Impact Ventures capital fund...patients who cannot get the drugs they want through local healthcare systems are using the organization to self-import medicines from abroad...