- FDA approves first drug in U.S. with digital ingestion tracking (reuters.com)Digital Pills That Talk to Your Doctor Are Here (wsj.com)
The U.S. Food and Drug Administration said...that it had approved Otsuka Pharmaceutical Co Ltd’s Abilify MyCite, the first drug with a digital ingestion tracking system to be approved in the United States...The product, which uses digital tracking to record if the medication was taken, has been approved for the treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar I disorder and for use as an add-on treatment for depression in adults...The system sends a message from the pill’s sensor to a wearable patch, which then transmits the information to a mobile application, so that patients can track the ingestion of the medication on their smartphone.
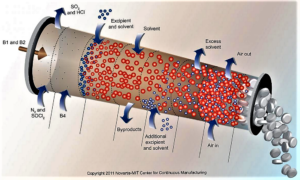
- Continuous Manufacturing: Pfizer, Vertex, AstraZeneca and Others Weigh FDA Plans (raps.org)
The US Food and Drug Administration has been encouraging the adoption of continuous manufacturing techniques...and several companies recently offered the agency some suggestions to refine its work around the developing technology...continuous manufacturing allows companies to move more seamlessly and efficiently...Last week, FDA finalized guidance on how manufacturers can participate in the agency’s program to advance continuous manufacturing...And in a blog post, Michael Kopcha, director of FDA's Office of Pharmaceutical Quality, pointed to Vertex's cystic fibrosis drug Orkambi (lumacaftor/ivacaftor) and Janssen's HIV treatment Prezista (darunavir) as examples of companies successfully using continuous manufacturing after engaging with FDA's emerging technology team...Vertex Pharmaceuticals...noted several contradictions in how batch size is described in an FDA document and sought further clarity and certainty regarding FDA’s understanding and expectations regarding batch size...AstraZeneca...asked if FDA might consider harmonizing the assessment process of the changes relating to continuous manufacturing, including a mechanism by which there could be some mutual recognition across countries participating in the International Council on Harmonization. AstraZeneca also said it "does not see the need" for a full ICH guideline on continuous manufacturing, which the company says FDA has been supporting. But the European drug industry group known as EFPIA is suggesting a Q&A document based on ICH Q8 and AstraZeneca says it "concurs with that approach."
- Manufacturers Seek Strategies for Ensuring Quality of Innovative Therapies (biopharminternational.com)
Continued industry investment in advanced biologics will raise further regulatory challenges and require innovative manufacturing systems...The challenges in producing cellular and gene therapies and other cutting-edge products that meet standards for safety, efficacy, and quality was high on the agenda for biopharmaceutical companies at PDA/FDA annual joint regulatory conference...
- Center for Biologics Evaluation and Research...is expediting the development and approval of human cellular and tissue products...vetting new regenerative medicine advanced therapies, including those utilizing gene-editing technology to treat malignancies and diseases...
- National Institutes of Health...cell biology and tissue engineering to advance regenerative medicine...these products require very different manufacturing and dosing processes and raise multiple challenges for scale-up and for keeping cells alive during processing...
- CBER’s Office of Tissues and Advanced Therapies...devising standards for characterization and potency assays for regenerative products, stem cell therapies, therapeutic vaccines, gene therapies, antivenins and certain combination products…importance of controlling the manufacturing process for these products and...challenges in achieving the right level of product characterization based on critical quality attributes.
- How to Protect a Drug Patent? Give it to a Native American Tribe (nytimes.com)Allergan and Saint Regis Mohawk Tribe Announce Agreements Regarding RESTASIS® Patents (srmt-nsn.gov)Mylan says Allergan misusing tribal sovereignty in patent dispute (reuters.com)Prevnar 13 among blockbusters industry watchers peg as tribal licensing candidates (fiercepharma.com)
The drugmaker Allergan announced...that it had transferred its patents on a best-selling eye drug to the Saint Regis Mohawk Tribe in upstate New York — an unusual gambit to protect the drug from a patent dispute...Under the deal, which involves the dry-eye drug Restasis, Allergan will pay the tribe $13.75 million. In exchange, the tribe will claim sovereign immunity as grounds to dismiss a patent challenge through a unit of the United States Patent and Trademark Office. The tribe will lease the patents back to Allergan, and will receive $15 million in annual royalties as long as the patents remain valid...The surprising legal move rippled quickly through the pharmaceutical world...setting off speculation about whether other drug companies would soon follow suit in order to protect their patents from challenges through a patent-review process that the industry despises...If Allergan succeeds in holding onto its patents, “we will probably see multiple branded companies housing their patents with Indian tribes...
- Smartphone-compatible ultrasound device gets FDA nod (pharmaphorum.com)
An iPhone-compatible ultrasound device that can deliver scanning for a fraction of the price of leading technologies, has been approved by the FDA...Traditionally, ultrasound scanners consist of three transducers connecting to large, bulky units which, when combined, carry a hefty price tag...The Butterfly iQ – the world’s first ‘ultrasound-on-a-chip’ device – houses all three of these transducers and over 10,000 sensors in a single handheld scanner, allowing for faster, easier, and cheaper ultrasound scanning...Doctors perform an ultrasound scan as they usually would with imagery appearing on their smartphone. The images are then sent to cloud storage, allowing for connectivity to hospital medical record systems...Offering a unique blend of affordability, diagnostic versatility, and assistive intelligence, Butterfly has the potential to impact human health more profoundly than any diagnostic device since the stethoscope, invented over 200 years ago. At less than $2,000, healthcare providers can purchase an easy-to-use, powerful, whole-body medical imaging system that fits in their pocket...
- Medical Technology Is Losing Share Of Venture Investments (forbes.com)
Medical technology continues to lose ground when it comes to all U.S. venture capital investment as value-based care takes hold of the healthcare system and the industry fights to get rid of a device tax...The share of medical technology venture deals dropped to just 4% of total deals last year compared to the industry’s 13% share 25 years ago...There were 420 medical technology venture deals in 2016 out of more than 10,000 total venture deals...medical technology companies had been paying a 2.3% medical device tax on sales under the Affordable Care Act until a two-year moratorium began in January 2016. Before the device tax was put on hiatus, the IRS collected between $1 billion and $2 billion a year in 2013, 2014 and 2015...This move away from fee-for-service medicine to value-based models means insurance companies don't always pay for the medical device a doctor wants to use. Purchasing of devices at large multi-hospital systems has shifted from doctors to “hospital purchasing committees,”.. medtech products...have digital health elements embedded into them...Inherently, digital health solutions are more solution-based...and they have the ability to measure outcomes...Whether medical technology companies can develop products that improve outcomes and measure them will be key...
- Challenge of Allergan tribal patent deal in uncharted legal territory (reuters.com)
As generic drug manufacturers are gearing up to argue that a deal Allergan Plc made with a Native American tribe to shield patents from administrative review is a sham, some experts say the generic companies are in uncharted legal territory...Last week, Allergan announced it would transfer the patent rights to its Restasis dry-eye treatment to the Saint Regis Mohawk Tribe, which will license them back to the company in exchange for ongoing payments...Richard Torczon, a lawyer for generic drug company Mylan NV, said the tribe is abusing the defense of sovereign immunity, which he said is intended to shield tribes that get dragged into court without their consent...“The tribe here has not been dragged into this proceeding against its will,” Torczon said during a hearing...before three judges from the patent board. “It has deliberately by its own admission targeted these proceedings for exactly this kind of revenue-generating opportunity,”
- Walmart uses robots to keep store shelves full (drugstorenews.com)
The discount giant is using a shelf-scanning robot to detect out-of-stock items, incorrect prices and wrong or missing labels. The initiative is an effort to automate tasks that are repeatable, predictable and manual for its associates, Walmart spokesman Justin Rushing said in the retailer’s blog...Using data and vision technology, the wheeled robot roams store aisles ready to simplify routine, but time-consuming tasks. On-hand robots are making it easier for personal shoppers to fulfill online orders, as well as freeing up associates to serve in-store shoppers...Jeremy King, chief technology officer for Walmart U.S. and e-commerce, said, “The robots are 50% more productive than their human counterparts, and can scan shelves significantly more accurately and three times faster.”...This is not Walmart’s first try at adding automated, robotic solutions. The discounter continues to expand its fleet of “Pickup Towers,” massive orange vending machines where shoppers can retrieve their online orders in less than a minute...The discounter was also recently granted a patent that would allow the chain to use drones to shuttle merchandise between departments and dedicated delivery locations within its stores...
- Apple Likes the Patent ‘Death Squad.’ Allergan Pays to Avoid It (bloomberg.com)
Allergan Plc’s decision to pay a Native American tribe $15 million a year rather than let one of its blockbuster drugs be scrutinized by the U.S. Patent & Trademark Office is part of a backlash against an agency review panel that has been dubbed a “death squad.”...The drugmaker earlier this month transferred ownership of patents protecting a medicine with $1.49 billion in sales last year to the Saint Regis Mohawk Tribe of upstate New York. The tribe, which will receive royalties every year, says that as a sovereign entity it is immune from such civil patent challenges....The creative -- and untested -- maneuver is designed to circumvent the Patent Trial and Appeal Board...critics say the board has made it too easy for rivals to attack patents and they’re pressing Congress, the courts and the patent office for changes...companies such as Google or Apple Inc., which are among the biggest users of the review board to fend off what they consider nuisance lawsuits from companies looking for a quick payday...the Supreme Court agreed to take a case to determine if the reviews are constitutional -- critics of the reviews say a patent is a property right that only federal courts can revoke. But even those who want to see the system dismantled say that case is a long shot...The patent office has been considering changes to its procedures...
- The FDA just approved the first app for treating substance abuse (cnbc.com)
Federal regulators...approved the first mobile app to help treat substance use disorders...The app, developed by a start-up called Pear Therapeutics, is designed to be prescribed by clinician and used alongside counseling...Pear's technology digitizes a form of talk therapy called cognitive behavioral therapy, or CBT, which focuses on "examining the relationships between thoughts, feelings and behaviors...Pear Therapeutics is part of a burgeoning category of health start-ups known as digital therapeutics. The idea is that software can improve a person's health, without the same cost and side effects of medical treatment...Pear's app has not been approved to treat opioid dependence, but...the company has developed a version of the software that is currently under submission. It's designed to be used alongside opioid replacement therapies.