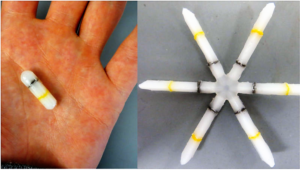
- Freaky Expanding Pill Stays in Your Gut for Days to Deliver Drugs (gizmodo.com)
The problem with pills is that you have to take them on a regular basis. An innovative new pop-up capsule solves this problem by staying in the stomach for days, where it slowly releases medication over the course of an entire treatment...This “ultra long-acting oral drug delivery” system was developed by researchers at MIT and healthcare firm Lyndra, and it’s changing the way we think about pills. After swallowing, the capsule unfurls into a star-like shape—a configuration that prevents it from entering the digestive tract, while still allowing food to pass. Then, for the next seven to ten days, it stays in the stomach, slowly releasing medication. The star eventually breaks down, allowing it to safely pass through the intestines…this pill will be particularly helpful in the developing world, and for those who have difficulty, for whatever reason, taking drugs as prescribed, including patients with hypertension, diabetes, neurological disorders, and opioid addiction.
- Buyers clubs for cheaper drugs help fight hepatitis and HIV (reuters.com)
Frustrated by the high price of antiviral drugs, thousands of patients from London to Moscow to Sydney are turning to a new wave of online "buyers clubs" to get cheap generic medicines to cure hepatitis C and protect against HIV infection...While regulators warn that buying drugs online is risky, scientific data presented at a recent medical conference suggest that treatment arranged through buyers club can be just as effective as through conventional channels...The buyers clubs' websites act as middlemen by providing details of trusted online pharmacies and drug manufacturers, exploiting a loophole in World Trade Organization patent rules that allows small-scale imports of medicines for personal use...the advent of today's buyers clubs is just the latest chapter in an ongoing war over drug prices...
- NACDS supports pilot of new pharmacy practice model (drugstorenews.com)
The National Association of Chain Drug Stores, in partnership with the Pharmacy Society of Wisconsin...announced plans to move forward with a new practice model to improve patient access to quality and efficient care, and advance pharmacy in a broader healthcare setting...The pilot project, "Advancing Community Pharmacy Quality: Leveraging Tech-Check-Tech to Expand Patient Care Services in Community Pharmacies," aims to promote better access to patient care. Specifically, the pilot will examine a new practice model that fosters collaboration across healthcare settings and employs an enhanced operational model. This new practice model holds promise in transforming the nation's healthcare system to deliver better access to clinical care by leveraging health care resources in a smarter way while ensuring patient safety and high operating standards...A key component of this model involves trained and validated pharmacy technicians completing the final check of a prescription filled by another technician, a pharmacy technician verification process known as tech-check-tech. This process allows pharmacists to reinvest the time saved by providing direct patient care services such as immunizations, disease state testing and medication therapy management, among others, which has a positive impact on overall patient outcomes...
- Survey: 97% plan to use digital health tech in trials over next five years (outsourcing-pharma.com)
According to survey results, published by Validic, more than 60% have used digital health technologies in clinical trials, and more than 97% plan to use such tools more over the next five years…Validic director of marketing told us...Medication adherence has always been a top priority for pharma, which is not surprising given the close correlation between participants’ compliance and the ability to get a drug to market faster and more cost effectively...Given advancements in technology, remotely tracking and monitoring prescription compliance has not only become a reality, but also an increased priority for pharma...As more evidence is available and sponsors continue to realize real-time, objective adherence data enables adaptive trial design and the ability to confidently make adjustments to protocols, we expect to see the interest in adherence technologies continue to rise…mobile applications have been a popular “entry point” for companies looking to being using digital health, “but we’re expecting to see greater use of wearables and sensors in the near-term...the respondents are most interested in reducing trial costs, while also being able to effectively demonstrate a drug’s efficacy in the real world...
- Pharm Exec’s 2017 Pipeline Report (pharmexec.com)
The industry is readying for a leap into a new age of complex therapies, as major advances seem mere steps away from market approval. Regenerative cell-based therapies, CAR-T and immuno-oncology combinations are just some of the fields where researchers are reaching for new heights that could alter the treatment paradigm. Elaborate manufacturing and rising drug costs, however, loom as deep chasms to cross.
- CAR-T cutting it close
- Combos, to name a few
- A PD-1 backbone?
- Targeted therapy: Is that still a thing?
- NASH players
- State of Alzheimer’s
- Moving fast in Zika
- An eye on complexity
- The verdict: A pipeline of puzzles
...As researchers blaze the meandering and thorny path toward curative treatments, a clearing is visible on the horizon. But to get there, the industry must confront a daunting chasm—making the previous generation’s small molecule-to-antibody transition look like an easy stride across a tame stream....
- AI Takes On Drug Safety (fortune.com)
IBM Watson Health tries to do what no pharma company has done: solve the drug-safety puzzle...Big Blue has found yet another business application for its precocious cognitive computing system. IBM Watson Health is collaborating with the biopharmaceutical company Celgene to develop a new platform for evaluating the safety of drugs—both before and after they hit the market—the two companies are announcing this morning. The new offering, "Watson for Patient Safety," will gobble up anonymized medical records, claims data, and millions of electronic submissions to the FDA about potential drug side effects (known as individual case safety reports) to see if it can learn about the hidden dangers of medicines before they become too costly...The problem is one of the toughest in drug development...
- MD Labs is part of a new industry called pharmacogenetics in Reno (nnbw.com)
Not everyone reacts to the same medication in the same way...Benedryl makes some people drowsy and others wired. Antidepressants have no affect on 38 percent of patients...Some people are more susceptible to addiction from pain medications while others get pain relief without getting hooked...MD Labs is part of a new industry called pharmacogenetics that uses genetics to map specific genes involved in the metabolism of and response to specific drugs...Ruttledge and Denis Grizelj, co-founder and CEO, began MD Labs in 2011 as a toxicology testing facility for physicians nationwide. They expanded to pharmacogenetic testing in 2014, with the development of their proprietary genetic test Rxight, which maps genes that affect more than 200 medications...Because it’s genetically based, Rxight is a once-in-a-lifetime test...Preemptive testing makes the patient’s genetically-based profile of drug reactions available to doctors and pharmacists before illness strikes and before a drug is prescribed...MD Labs contracts with Saint Mary’s Health to offer the testing and consultation at both Saint Mary’s Regional Medical Center and Saint Mary’s Medical Group Primary Care Northwest Reno...Pharmacists at those locations are Rxight Certified to administer the test and provide consultation...Nationwide the company has 100-110 employees total, which includes about 55 people in the Reno office plus a sales force in offices in Chicago and Pennsylvania...MD Labs owners see a future in which pharmacogenetics testing is routine.
- Zipline raises $25M as it prepares to launch drone delivery medical supply service in U.S. (medcitynews.com)
Zipline, a San Francisco Bay-area company that’s using drones to deliver medical supplies such as blood, (and soon, medication and vaccines) to rural areas and in developing countries, has closed a $25 million Series B round...The funding comes at a time when Zipline is expanding its business in Rwanda and preparing to launch in the U.S...The drone business is planning to launch its drone delivery service in the U.S. next year, as part of a partnership with the White House and the U.S. Federal Aviation Administration...Zipline has been ramping up its medical drone business in Rwanda. The company signed a one-year partnership with Rwanda’s government to deliver blood for transfusions to the western half of the country and plans to start deliveries in the eastern half of Rwanda by the end of the year.
- These pricey cholesterol drugs aren’t selling. And that has the biotech industry sweating (statnews.com)
It looked like a surefire way to make billions...A year ago, two new drugs (Repatha and Praluent) that used a novel mechanism to drive down cholesterol levels came on the market, and were promptly crowned as blockbusters in waiting. Analysts estimated sales at more than $3 billion a year...But the two drugs have been commercial flops, in part due to a complicated reimbursement system that has frustrated doctors, confused patients, and left the biotech industry worried about the implications for other high-priced drugs in the pipeline...The failures could send a chill through the still-booming biotech business, which relies on the idea that the risky, expensive process of developing new drugs can one day pay off big... some doctors are hesitant to prescribe them until there’s more information...Another hurdle: Getting insurers to pay for the drugs, which both have list prices of about $14,000 a year...the squabble over cost, access, and availability is unlikely to end any time soon. And the fear among biotech insiders is that the commercial disappointment of PCSK9 therapies will imperil new cardiovascular therapies...
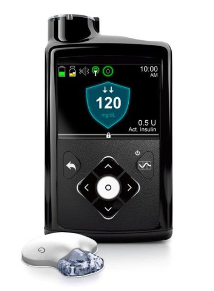
- FDA approves 1st ‘artificial pancreas’ for type 1 diabetes (upi.com)
The Food and Drug Administration...approved the first automated insulin delivery system -- a so-called "artificial pancreas" -- for people with type 1 diabetes...This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin…The device -- Medtronic's MiniMed 670G -- is what's known as a hybrid closed-loop system. That means it monitors blood sugar and then delivers necessary background insulin doses. The device will also shut off when blood sugar levels drop too low...this device isn't yet a fully automated artificial pancreas. People with type 1 diabetes will still need to figure out how many carbohydrates are in their food, and enter that information into the system, the agency noted..