- Valeant’s big pharmacy deal with Walgreens includes quirky drug-buyback plan (fiercepharma.com)Valeant Taking Titles to Drugs on Walgreens’ Shelves (sub req) (wsj.com)
Valeant Pharmaceuticals' much-ballyhooed agreement with Walgreens includes an unusual inventory provision that helps explain why the pharmacy chain might have been eager to strike a deal...Valeant is buying back its drugs from Walgreens and selling them on consignment to the pharmacy, a provision that triggers an upfront financial hit. Walgreens won't physically return the drugs, just the title to them...It's just one out-of-the-ordinary feature of an out-of-the-ordinary pharmacy deal. Valeant announced last week that it had teamed up with Walgreens to distribute its drugs, discounting key skin and eye brands by 10% and another slate of meds by an average of 50%. Walgreens is taking the Valeant drugs on consignment and collects fees for filling prescriptions…The Walgreens partnership replaces Valeant's relationship with Philidor, a specialty pharmacy that was closely tied to the drugmaker until questions arose about its operations...The drugmaker is expecting a sales hit from the pharmacy shift, partly because of the mechanics of the Walgreens deal and partly because of lower demand for its drugs in the wake of the Philidor allegations.
- Two serialization companies team up to tackle counterfeit pharmaceuticals (americanpharmacynews.com)Prohibition on proactive serialization for the EU FMD (securingindustry.com)
Systech International and Servicepoint recently joined forces to provide serialization and automation solutions to the pharmaceutical manufacturing industry...Internationalization, outsourcing, e-tailing and the expansion of international trade zones have created enormous complexities throughout the supply chain and product life cycle...This has resulted in a rapid escalation of global counterfeiting issues, threatening consumer safety like never before...Systech is known to have pioneered serialization and is becoming a model for the future of authentication. It unifies and protects the supply chain through...authentication and track and trace technologies that ensure regulatory compliance and reduce risks. These technologies have been used not only in the pharmaceutical industry, but also in industries, such as life science and consumer packaged goods...As part of a regulation adopted by the European Commission in October, the serialization of prescription medicine packages will be mandatory in all European Union countries by 2019. There are currently 15,000 prescription medicine production lines in Europe, and some may have to be either replaced or automated in order to put serialization into effect.
- Unlocking my genome: Was it worth it? (cnbc.com)
...I discovered, it was also unlikely I'd learn something particularly useful, or "actionable," as geneticists describe it, a dominant mutation that would predispose me to a treatable cancer or heart disease, for example...I learned, because we're still very much in the early days of interpreting our own genetic information...There is a great deal of debate about whether this is worth sequencing...because it hasn't been proven that you can change something and change their outcome…Our understanding of all the implications of our genetics hasn't caught up yet to the power of sequencing technology...I only had one result under "clinically significant findings" in the report...I carry one copy of a mutation called Factor V Leiden...your blood actually clots a little bit faster than people who do not have this mutation...my results included a pharmacogenomics report analyzing how my genes indicate I may react to 12 different drugs, from blood thinners like warfarin and Plavix to the cholesterol-lowering medication Zocor...Many believe this will be among the nearest-term applications of genome sequencing in medicine, using our genetic signatures to improve how we match the right drugs to the right patients...within eight to 10 years, it will be routine for healthy people to have their genome sequenced, and for that information to be a regular part of every medical encounter. Between now and then, costs are expected to continue to come down, reimbursement by insurers is expected to gain more clarity, and more proof is expected about how useful personal genome sequencing can be…For most of us, genes aren't our destiny. They're a blueprint for how we start out, and then life plays a major role.
- Ibuprofen gels not a patch on new delivery tech say UK developers (in-pharmatechnologist.com)
UK researchers have developed an ibuprofen patch they claim offers better dosage control than gel formulations of the pain drug…The patch – which was developed by researchers at the University of Warwick and spinout company Medherant – consists of a transparent layer that is stuck to the skin with an adhesive polymer into which…in this case ibuprofen - is incorporated… the approach enables precise dosage control because the patches have “a defined size with a set amount of drug.”…the technology has wider application…“We know that a lot of other APIs can be incorporated in our patches”…Medherant is interested in partnering with the pharmaceutical industry.
- Which Biotechnologies Were Hyped (And Which Went Out Of Favor) In 2015 (forbes.com)
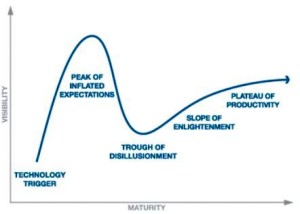
"Don’t Believe the Hype."...It’s good advice for people operating in the R&D side of pharmaceuticals. Many exciting things are happening today in the science, so it’s easy to get carried away. But this year’s market correction–driven in part by systemic concerns about drug pricing, a few clinical trial blowups and shenanigans—should serve as a vivid reminder. No matter how good the fundamentals are, biotech still hasn’t figured out how to carefully calibrate perceptions to keep the steam engine steadily chugging along, without letting things get too hot or too cold...Hype has always been part of biotech because investments must be made on glimmers of promise, otherwise known as incomplete data. Without hype and hope, the industry couldn’t exist...There’s no good way to quantify perceptions, and things change fast in biotech, but I do talk every day to lots of different people with their fingers on the pulse of different things. So I thought it would be fun to try again this year with plotting various biotech platforms and concepts along the Gartner hype curve.
Peak of inflated expectations
- CRISPR/Cas9 genome editing.
- Microbiome everything.
- Genomic wellness.
- Conquering antibiotic resistance.
Trough of Disillusionment
- Digital health.
- Stem cell therapies.
Climbing up the Slope of Enlightenment
- Immuno-oncology.
- Viral Vector Gene Therapy.
- Long-read sequencing.
- FDA launches Web-based precision medicine platform for next-generation sequencing (healthcareitnews.com)FDA Launches precisionFDA to Harness the Power of Scientific Collaboration (blogs.fda.gov)
Food and Drug Administration...launched the beta version of precisionFDA, its new collaborative platform for the exploration of next-generation gene sequencing...First announced in August, the platform features more than 20 public and private sector participants…Next-generation sequencing enables researchers to compile a vast amount of data on a person's exact order or sequence of DNA...scientists can look for meaningful differences in DNA that can be used to suggest a person's risk of disease, possible response to treatment and assess their current state of health. Ultimately, what we learn about these differences could be used to design a treatment tailored to a specific individual...The hope is to grow this community and improve the usability of precisionFDA in the coming months and years...One way we'll achieve that is by placing the code for the precisionFDA portal on the world's largest open source software repository, GitHub, so the community can further enhance precisionFDA's features...
- Make Doctor’s Licenses Like Driver’s Licenses? Medical Groups Say No (realclearhealth.com)
A doctor licensed in one state who wants to practice in another still needs a license from the other state. That’s a costly and time-consuming process, especially in an era when many health plans and their employees operate across state lines and the use of telemedicine, in which patients and their providers interact from a distance, is growing...But the state licensing situation for doctors...is starting to change...Doctors...are creating their own multistate compact...it will provide an expedited application process to get licenses in other states...The main obstacle to portability for all the professions is that they are regulated by the states, each of which is free to demand its own qualifications — and collect its own fees — for licensing. To achieve portability, states either have to standardize their licensing requirements or agree to live with the differences, so long as there are some baseline qualifications accepted by all...doctors have created a compact that the Federation of State Medical Boards says will offer an expedited and cheaper process for getting a license to practice in member states. So far, 11 states have joined the compact, with nearly an equal number expected to join next year. Telemedicine advocates and doctors who use telemedicine say the doctors’ portability model, which still requires full licensure in every state, will impede the spread of telemedicine, particularly in rural areas that have a shortage of doctors.
- Push Is On for Medical Records Transparency (realclearhealth.com)Foundations Unite to Support Access to Clinical Notes for 50 Million Patients Nationwide (opennotes.org)
Perched on an exam table at the doctor’s office watching the clinician type details about their medical problems into their file, what patient hasn’t wondered exactly what the doctor is writing? As many as 50 million patients may have a chance to find out in the next few years, following the announcement this week of $10 million in new grants to expand the OpenNotes project, which works with medical providers to expand patient access to clinician notes...OpenNotes started in 2010 as a research project to examine what would happen if patients had easy access to their doctor’s visit notes, which may include a summary of their conversation, the symptoms patients describe and their doctor’s findings from a physical exam. Although patients have a legal right to their medical records, getting those documents is often difficult and expensive...Our goal is basically to make fully transparent medical records the standard of care across the country...The project works with providers, patient groups and electronic health record vendors, among others, to encourage the use of OpenNotes, and evaluates the impact of OpenNotes on health outcomes and costs...In addition to improving communication between patients and their providers, some research suggests OpenNotes may improve patients’ ability to stick with their medication regimens...
- 7 key ingredients in Celgene’s recipe for biopharma dealmaking (medcitynews.com)
Over the past seven years, Celgene has emerged as one of the most active and creative deal-makers in the biopharma industry...The Boston Consulting Group’s Biopharma Partnering Survey and Benchmarking analysis examines BD activity and perceptions across the industry...Celgene scored as the best partner on 78% of the partnering and culture metrics (7/9), as perceived by the sell-side biotechs...here are the “seven habits”, or more aptly just attributes, of Celgene’s...successful external R&D strategy, spanning their...leadership context and...culture:
- Exhibiting more David, less Goliath.
- Starting with a clean sheet of paper.
- Embracing risk, empowerment, and trust.
- Governing with unconventional out-of-the-box leadership.
- Inverting and resizing the talent model.
- Learning at every opportunity.
- Living better through (personal) chemistry.
- 300 million child-friendly antimalarial treatments supplied without profit by Novartis (malaria.novartis.com)
Novartis announced today that it has reached a delivery milestone of 300 million pediatric antimalarial treatments supplied without profit since 2009, helping to reduce the disease burden for children in more than 30 malaria-endemic countries. Coartem® Dispersible is the first artemisinin-combination therapy developed by Novartis in collaboration with Medicines for Malaria Venture specifically to meet the needs of children. Never before have so many pediatric treatments been distributed in such a short timeframe to children suffering from malaria…"This milestone underscores our long-standing commitment to the fight against malaria and to the children who are most at risk from the disease…"We are proud of the part we have played in helping to reduce childhood deaths from malaria. And we continue to provide medicine at no profit to people who need it, contributing to the goal of a world free from the disease."