- Bumper haul of expensive new drugs heads to U.S. and Europe (reuters.com)
Food and Drug Administration has so far approved 37 novel drugs in 2015, more than the 34 that had been cleared by this stage a year ago and just short of 2014's final total of 41…European Medicines Agency is also waving through more products, recommending a total of 84 new medicines so far, up from 75 in the first 11 months of 2014…The brisk pace of new arrivals over the past two years reflects improved productivity in drug research labs and a change of pace by regulators, who have committed to speed up the process of getting life-saving treatments to patients, especially in cancer…The science has got better and we seem to be finding more molecules that are showing material improvements…the rapid pace of new drug launches is forecast to continue, with 225 new drugs expected to be approved between 2016 and 2020…Drug companies argue they need to make decent profits to pay for the billions of dollars needed for drug research. Many companies also have extensive low-cost or even free access schemes for patients who cannot afford their medicines… For healthcare systems in the developed world, paying for such pricey medicines is a challenge - but for many patients in poor countries they will remain out of reach, reflecting the economic realities of drug development.
- FDA approves Adapt Pharma’s nasal spray for opioid overdose treatment (reuters.com)
Food and Drug Administration approved the first-ever nasal spray emergency treatment for opioid overdose…The spray, developed by privately held Adapt Pharma Ltd, uses naloxone, a drug used to treat opioid overdose for nearly 45 years but approved only in injectable forms…The treatment…is expected to have wide coverage under health insurance with affordable co-pays…Group purchasers, such as law enforcement, fire fighters, departments of health, local school districts, colleges and universities, and community-based organizations will be able to purchase the spray at a discounted price of $37.50 per 4 mg device…
- Why FDA Should Oversee Laboratory Developed Tests (blogs.fda.gov)The Public Health Evidence for FDA Oversight of Laboratory Developed Tests: 20 Case Studies (fda.gov)Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs) DRAFT GUIDANCE (fda.gov)Theranos isn’t the only diagnostics company exploiting regulatory loopholes (theverge.com)
Today FDA is issuing a report that illustrates the real and potential harms to patients and to public health from certain laboratory developed tests (LDT) – tests that are designed, manufactured and used in a single laboratory…But times have changed. LDTs have increased in complexity and availability and are now frequently used to diagnose common, serious medical conditions, including cancer and heart disease, with potentially greater impact on patients...LDTs are still under a general policy of enforcement discretion. That means they have rarely undergone FDA review to determine whether they are accurate, reliable, and provide clinically meaningful results...FDA’s own adverse event reporting databases rarely capture problems associated with a faulty LDT...the Agency was able to pull together 20 case studies based on information available in the public domain that show how lack of LDT oversight may be causing or is causing significant harm to patients…FDA has proposed to step up our oversight of LDTs. We issued a draft guidance last year which we’re currently working to finalize, that proposes to phase in enforcement of premarket review requirements for LDTs. FDA oversight would help ensure that tests are supported by rigorous evidence, that patients and health care providers can have confidence in the test results, and that LDTs have more scientifically accurate product labeling.
- The Future Of Medicine Might Be 3D-Printed Pills (itechpost.com)
Researchers from the University of Columbia, University of North Carolina, and Wake Forest University presented a 3D printer software that creates pills for patients. The software calculates the medicine's dosage based on the patient's medical and biological data…a prototype software that creates customized pills for patients. The software uses algorithms that enables it to adjust medical dosages based on patient information like weight, race, liver and kidney functions…In a trial run, the researchers created patient profiles that resulted in different dosages of 80 total pills printed. The pills ranged from 124 mg to 373 mg, which were dosages with high accuracy with little inconsistency.
- Pharmacy Podcast – Pharmacy Cloud Accounting Technology – Sykes & Company (pharmacypodcast.com)
We are joined by independent pharmacy business accounting expert – Ollin Sykes founder of Sykes & Company, P.A. (podcast 21:45 min)
- Cheating On Court-Ordered Drug and Alcohol Tests Just Got A Lot Harder (kolotv.com)
Offenders facing court-ordered drug tests in Reno Municipal Court have often found ways around the system. That just got a lot harder and consequences for cheaters a lot swifter…Their crimes may vary…But the common underlying cause is substance abuse. That makes them potential candidates for one of the specialty courts. Instead of housing them behind bars, they are sentenced to sobriety and a rigorous schedule of random testing… But the court now has a new drug-screening lab. Samples, as many as 54 at a time, can be analyzed for 11 kinds of drug or alcohol content. Accurate results in minutes…The lab is the first of its kind in northern Nevada.
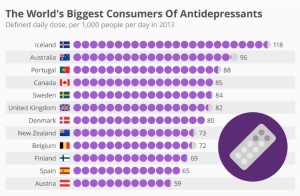
- The World’s Biggest Consumers Of Antidepressants (forbes.com)
Iceland is the biggest consumer of antidepressants worldwide, according to recent OECD (Organisation for Economic Cooperation and Development) report entitled “Health at a Glance 2015.” Some 118 out of every 1,000 Icelanders now consume these drugs on a daily basis, though the trend certainly isn’t new...Some experts attribute the country’s use of these drugs to a weakening of social taboos, along with a greater tendency to seek treatment. Some also believe there is a link between antidepressant consumption and the failure of all three of Iceland’s main banks during the financial crisis. Australia is in second position with 96 out of every 1,000 people taking antidepressants daily, while Portugal rounds off the top three with 88 per 1,000.
- Apixio launches cognitive computing platform (healthcareitnews.com)
Apixio announced…the release of its new cognitive computing platform, Iris, which it says will bring advanced data insights to healthcare by extracting and analyzing medical data previously trapped in electronic health records…The U.S. annually produces 1.2 billion clinical care documents, but about 80 percent of the data is unstructured and difficult to access…"Making sense of unstructured healthcare data is extremely challenging and requires sophisticated technology like cognitive computing to make the information useful,"…Iris is meant to give healthcare institutions access to patient data to create a more accurate care profile, thus improving the quality and efficiency...
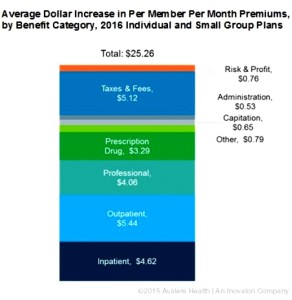
- Despite rhetoric, new data shows medicines are not primary driver of premium increases (catalyst.phrma.org)
Despite claims from insurers about the impact of medicine costs on premium increases, their own data indicates otherwise. In fact, just $3.29 of the average $25.26 increase in monthly premiums in 2016 is due to prescription drug costs, according to new research released yesterday by Avalere Health. So what is driving premium increases? Avalere found that the largest driver of premium increases was hospital services…analysis is based on actuarially-certified analysis submitted by the plans themselves to justify their premium rate increases. This data shows that prescription drugs dispensed at a pharmacy represent a smaller share of premium increases than inpatient hospitalization, outpatient hospitalization, professional services or taxes and fees. In fact, even if plans did not anticipate any increase in prescription drug spending, the average premium would still increase by more than $20 per month.
- Keystroke logger detected on hospital’s computers (healthcareitnews.com)
A hospital in Kentucky is notifying patients of a security incident, after it was discovered that some of its computers had been infected with a keystroke logger designed to capture and transmit data as it was typed…Muhlenberg Community Hospital had detected the malware on some of its machines…Affected computers were used to enter patient financial data and health information, potentially including names, addresses, telephone numbers, birth dates, Social Security numbers, driver's license/state identification numbers, medical and health plan information, financial account numbers, payment card information and employment-related information. Additionally, some credentialing-related information for providers may also be impacted…officials did say that they believe the malware could have captured username and password information for accounts or websites that were accessed by employees, contractors or providers using the affected terminals.