- This Week in Managed Care: November 30, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- November 23 Pharmacy Week in Review: FDA Approves Rifamycin for Travelers-related Illness, Study Finds Incidence of Eczema is Much Higher Than Other Inflammatory Conditions (pharmacytimes.com)
Nicole Grassano, PTNN, Pharmacy Week in Review, this weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- This Week in Managed Care: November 16, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network..
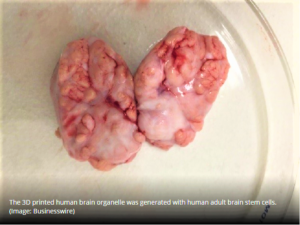
- 3D printed brain helps characterize 11 lead compounds, neuropathological research (outsourcing-pharma.com)
The stem cell research and therapeutics company Celprogen has identified and characterized 11 lead compounds for potential drug candidates for the treatment of Alzheimer’s, Parkinson’s, and Glioblastoma using its 3D model system...The 3D model system is unique since it represents the data generated closer to in-vivo data, eliminates animal studies, and represents patient’s brain physiological format...poor animal study design and reporting have raised concerns about whether current processes are the best way to conduct effective and efficient drug development...3D model systems aim to better inform risk-based decisions in drug development...
- November 30 Pharmacy Week in Review: Trial Evaluating Effectiveness and Safety of Drugs Used to Treat Patients with Ebola (pharmacytimes.com)
Nicole Grassano, PTNN, Pharmacy Week in Review, this weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Britain’s Storied National Health Service Is Chasing A Tech Upgrade (forbes.com)
The British take enormous pride in their National Health Service…Get sick in Britain and you can see a doctor, get a scan or even have surgery for free...Unfortunately the system also depends on tax money that can’t keep pace with an ageing population who need greater care than ever. Its deficit is estimated to be closing in on £1 billion ($1.3 billion)...Many believe technology can make the NHS more efficient, and so it has partnered with private companies...to serve NHS patients at a lower cost, by connecting them with doctors on a video call or even an automated symptom checker...Some worry that such deals spell the slow-and-steady privatization of the NHS and a move (God forbid) towards a system that looks more like that of the United States. But Hancock (Matt Hancock - health secretary) believes these partnerships are necessary if the NHS is going to survive.
- California, Massachusetts go toe-to-toe for title of best biotech hub (biopharmadive.com)
...the California Life Sciences Association released its industry report...pitting itself again against Massachusetts for the title of being the best state for the pharmaceutical industry...the report found California-based organizations received $3.9 billion in National Institutes of Health grants this year, ahead of Massachusetts, which was second in the nation at $2.7 billion. Additionally, CLSA found California companies had more than 1,300 medicines in the pipeline and roughly 130,000 employees working for 1,500 biopharmaceuticals and medical device companies in 2018..the MassBio report found Massachusetts-based biotechs dominated the initial public offering market last year, making up nearly half of all biotech IPO money and more than a third of biopharma venture capital funding...
- This Week in Managed Care: November 23, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- FDA plan would ease regulations for prescription drug apps (biopharmadive.com)
The Food and Drug Administration is seeking public comment on a proposed framework for regulating software applications developed by drugmakers for use in conjunction with their prescription drug products...The new approach would treat most prescription drug apps, including dose calculators, symptom trackers and medication reminders, as promotional labeling...drugmakers would need only to submit to the agency copies of the content of what the apps display to consumers, following existing reporting requirements for promotional materials...In other cases, such as when a drugmaker wants to show that software has an effect on a clinical outcome and wants to include information about the software in the FDA-required drug labeling, prior FDA approval would be required...
- November 16 Pharmacy Week in Review: Emergency Ebola Fingerstick Test with Portable Reader Receives Authorization (pharmacytimes.com)
Laura Joszt, welcome to the Pharmacy Times News Network, Pharmacy Week in Review, , this weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.