- This Week in Managed Care: March 16, 2018 (ajmc.com)
Kelly Davio, This Week in Managed Care from the Managed Markets News Network
- Drug copays sometimes exceed costs (reuters.com)Frequency and Magnitude of Co-payments Exceeding Prescription Drug Costs (jamanetwork.com)Patients Overpay For Prescriptions 23% Of The Time, Analysis Shows (khn.org)
Insurance companies may be asking people to shell out more money for drug co-payments than the drugs actually cost, a new study suggests...Generic drug co-payments in the U.S. exceeded the cost of medicines about 28 percent of the time – or for more than one in four prescriptions, researchers found...Co-payments for branded drugs were higher than the medication cost about 6 percent of the time, they report in JAMA...“This is money that patients could be saving if they knew about and could avoid the practice,” said lead study author Karen Van Nuys of the Schaeffer Center for Health Policy and Economics at the University of Southern California...To avoid overpayments, patients should always ask the pharmacist if their costs would be lower if they paid cash instead of using their insurance...“Pharmacists might not be allowed to offer this information to patients due to `gag clauses’ but if patients ask, pharmacists can tell them,”...“Several states have banned these practices and allow pharmacists to offer this information but even if you live in one of those states, you should ask,”...“Patients can also use pricing tools on the internet like GoodRx.com to see what prices they could expect across a variety of pharmacies if they paid cash.”
- Pharmacy Week in Review: March 2, 2018 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
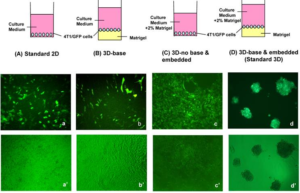
- Cancer research breakthrough shows a better way to predict drugs that will work (cnbc.com)Patient-derived organoids model treatment response of metastatic gastrointestinal cancers (science.sciencemag.org)
Cancer patients need time and drugs that, over time, are effective. They might have more of both according to the results of a new study...The study...showed that the creation of what the researchers are calling microtumors can help predict drug effectiveness in cancer patients better than the current standard method of testing the drugs on rodents. Researchers took biopsies from 71 colorectal cancer patients and made "cancer organoids," or cell culture models of cancerous organs. Researchers then treated these microtumors with drugs and observed the effectiveness in the laboratory...The microtumors grow in a 3-D matrix called a matrigel, and the whole process only takes six to eight weeks...If doctors can more accurately predict what drugs will treat someone's cancer, they can select the right drugs for the right patients. If they know for sure what drugs won't work, they can spare patients the painful side effects..."You can avoid unnecessary toxicity to patients who won't benefit,"..."You can switch to another treatment”...
- Pharmacy Week in Review: March 16, 2018 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- This Week in Managed Care: March 9, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Big pharma, big data: why drugmakers want your health records (reuters.com)
Drugmakers are racing to scoop up patient health records and strike deals with technology companies as big data analytics start to unlock a trove of information about how medicines perform in the real world...Studying such real-world evidence offers manufacturers a powerful tool to prove the value of their drugs...Real-world evidence involves collecting data outside traditional randomized clinical trials, the current gold standard for judging medicines, and interest in the field is ballooning...The ability to capture the experience of real-world patients, who represent a wider sample of society than the relatively narrow selection enrolled into traditional trials, is increasingly useful as medicine becomes more personalized...It’s getting more expensive to do traditional clinical trial research, so industry is looking at ways it can achieve similar goals using routinely collected data...The thing that has made all this possible is the increasing digitization of health records...Food and Drug Administration...believes more widespread use of real-world evidence (RWE) could cut drug development costs and help doctors make better medical choices...Under the 21st Century Cures Act, the FDA has been directed to evaluate the expanded use of RWE...As the breadth and reliability of RWE increases, so do opportunities for FDA to also make use of this information...
- Participants in rogue herpes vaccine research take legal action (fiercepharma.com)
Three people injected with an unauthorized herpes vaccine by a Southern Illinois University researcher have filed suit against his company, demanding compensation for alleged adverse side effects from the experiments...SIU professor William Halford, who died in June, had injected Americans with his experimental herpes vaccine...in 2016 and 2013 without safety oversight that is routinely performed by the FDA or an institutional review board...The lawsuit, which was filed Friday in an Illinois circuit court, demands compensation from Halford’s company, Rational Vaccines, alleging his research violated U.S. and international laws aimed at protecting the rights of participants in experiments…Rational Vaccines has said it considers the 2016 trial a success—though it is unclear what data it used to support that claim...SIU has...acknowledged that Halford’s conduct violated university rules and U.S. laws but said that Halford hid his misconduct from the university.
- This Week in Managed Care: March 2, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- FDA to Launch New Pilot Program for Orphan Designation Requests (raps.org)
With more than 700 orphan designation requests last year, the Food and Drug Administration...announced a new pilot program to make the request process more efficient...The pilot will include a new form intended to make the submission process easier for sponsors to complete orphan designation requests, and to make the process more efficient for FDA...FDA also released an on-line tutorial to guide sponsors through the submission process and Gottlieb noted there is a new inter-center consult process to streamline and standardize communications...In addition to the pilot, FDA vowed to eliminate the orphan drug designation backlog, and is planning a public meeting to discuss the scientific and regulatory issues related to cancer treatments that target a tumor’s genetic features rather than its location in the body...“We’ll also consider the appropriate application of orphan incentives to this new paradigm of drug development, and how we apply designations to these indications,”...