- FDA approves first blood test to help diagnose concussions (statnews.com)
The Food and Drug Administration gave a green light...for the first time to a blood test that doctors can use to help rule out concussions...The Brain Trauma Indicator, marketed by Banyan Biomarkers Inc., measures the levels of two proteins — called UCH-L1 and GFAP — whose elevated presence suggests a certain type of brain damage normally only visible on a CT scan. The test takes three to four hours, and doctors could use it to determine which patients need a CT scan to confirm the damage and which patients can rest easy...FDA Commissioner Scott Gottlieb said that this product could save the health care system money by preventing unnecessary neuroimaging tests. Additionally, by sparing some patients CT scans, it would reduce the radiation exposure associated with those scans.
- This Week in Managed Care: February 23, 2018 (ajmc.com)
Laura Joszt, Managing Editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Pharmacy Week in Review: February 16, 2018 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Pharmacy Week in Review: February 9, 2018 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
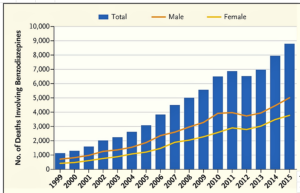
- Benzodiazepines: our other prescription drug epidemic (statnews.com)Perspective: Our Other Prescription Drug Problem (nejm.org)
“Benzos” is shorthand for benzodiazepines, a class of drugs often used to treat anxiety and insomnia. The dozen or so different types include Ativan, Klonopin, Valium, and Xanax...More people than you might think are taking them (three benzodiazepines are in the top 10 most commonly prescribed psychotropic medications in the United States). Yet few people realize how many people get addicted to and die from them...Between 1996 and 2013, the number of adults who filled a benzodiazepine prescription increased by 67 percent, from 8.1 million to 13.5 million. Unlike opioid prescribing, which peaked in 2012 and has decreased nearly 20 percent since then, benzodiazepine prescribing continues to rise. The risk of overdose death goes up nearly fourfold when benzodiazepines are combined with opioids, yet rates of co-prescribing benzodiazepines and opioids nearly doubled between 2001 and 2013. Overdose deaths involving benzodiazepines increased more than sevenfold between 1999 and 2015...
- FDA’s Scott Gottlieb wants to use funding boost to create a Center of Excellence on Digital Health (fiercehealthcare.com)
The Food and Drug Administration plans to use a proposed $400 million boost in federal funding to focus on a range of innovative approaches to speed the approval of new medical devices and create a new center that would support digital health oversight and address cybersecurity concerns...FDA Commissioner Scott Gottlieb, announced...That would include specific carve-outs planned for a new Center of Excellence on Digital Health and furthering the agency’s ability to use EHR data to evaluate medical devices...The Center for Excellence on Digital Health would oversee a revamped regulatory paradigm created through the FDA’s new software precertification program launched with nine companies in September. But the center would also create a cybersecurity unit to “enhance its ability to coordinate device-specific responses to cybersecurity vulnerabilities and incidents.” Over the past several years, medical device cybersecurity has emerged as particular concern for industry and regulators...Gottlieb also highlighted an expanded effort to integrate real world data into pre-market and post-market reviews of drugs and medical devices. The additional funding would allow FDA to develop analytic tools and pull real-time data out of EHRs associated with at least 10 million individuals across a range of healthcare settings...“Toward these ends, an expanded use of natural language processing for the assessment of information submitted to the agency would be developed in an effort to markedly speed recognition and remediation of emerging safety concerns,” he said. “The effort would cover a broad range of medical products, including drugs, biologics and medical devices.”
- Nordic project takes ‘manufacturing-on-demand’ approach to future drugs (in-pharmatechnologist.com)
Nordic Universities will investigate 3D printing, electrospraying, and microfluidics in an industry supported collaboration aimed at revolutionising production in an age of personalised medicine.
The collaboration, known as Nordic POP (Patient-Oriented Products), will use 35m DKK ($6m) of funding from NordForsk...to create flexible and translational approaches to personalised medicine manufacturing…Jukka Rantanen...University of Copenhagen...said that new patient-oriented and personalised drug products require a “totally new mindset” in the drug development process...“The project team is aiming to create new innovative drug products, where the dose, release mechanism, and size/shape of the product could be easily personalised based on the patient needs...“Instead of one-size-fits-all medication, the potential of new patient oriented products considering gender, age, lifestyle, genetic profile, metabolic capacity, and microbiota will be explored,”...
- Pharmacy Week in Review: February 23, 2018 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- This Week in Managed Care: February 16, 2018 (ajmc.com)
Laura Joszt, assistant managing editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
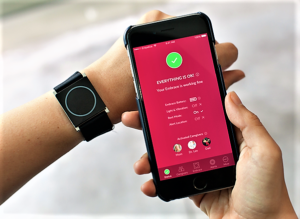
- FDA Clears the First Smart Watch for Use in Neurology (ptcommunity.com)
Wearable device identifies convulsive epileptic seizures and sends alerts to caregivers...The FDA has cleared the Embrace smart watch (Empatica, Inc.) for use by patients with epilepsy. Embrace uses advanced machine learning to monitor for the most dangerous kinds of seizures, known as “grand mal” or “generalized tonic-clonic” seizures, and sends an alert to summon caregivers’ help...The smart watch stands apart from any seizure detection system in that it measures multiple indicators of a seizure. Its unique property is its use of electrodermal activity, a signal used by stress researchers to quantify physiological changes related to sympathetic nervous system activity, also known as the "fight-or-flight" response. Embrace has been approved in Europe as a medical device for seizure monitoring and alert since April 2017.