- This Week in Managed Care: October 27, 2017 (ajmc.com)
Kelly Davio, welcome to This Week in Managed Care from the Managed Markets News Network
- This Week in Managed Care: October 20, 2017 (ajmc.com)
Kelly Davio, welcome to This Week in Managed Care from the Managed Markets News Network
- Week in Review: October 13, 2017 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Continuous Manufacturing: Pfizer, Vertex, AstraZeneca and Others Weigh FDA Plans (raps.org)
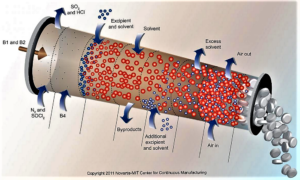
The US Food and Drug Administration has been encouraging the adoption of continuous manufacturing techniques...and several companies recently offered the agency some suggestions to refine its work around the developing technology...continuous manufacturing allows companies to move more seamlessly and efficiently...Last week, FDA finalized guidance on how manufacturers can participate in the agency’s program to advance continuous manufacturing...And in a blog post, Michael Kopcha, director of FDA's Office of Pharmaceutical Quality, pointed to Vertex's cystic fibrosis drug Orkambi (lumacaftor/ivacaftor) and Janssen's HIV treatment Prezista (darunavir) as examples of companies successfully using continuous manufacturing after engaging with FDA's emerging technology team...Vertex Pharmaceuticals...noted several contradictions in how batch size is described in an FDA document and sought further clarity and certainty regarding FDA’s understanding and expectations regarding batch size...AstraZeneca...asked if FDA might consider harmonizing the assessment process of the changes relating to continuous manufacturing, including a mechanism by which there could be some mutual recognition across countries participating in the International Council on Harmonization. AstraZeneca also said it "does not see the need" for a full ICH guideline on continuous manufacturing, which the company says FDA has been supporting. But the European drug industry group known as EFPIA is suggesting a Q&A document based on ICH Q8 and AstraZeneca says it "concurs with that approach."
- Week in Review: October 27, 2017 (pharmacytimes.com)
Nicole Crisano, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Week in Review: October 20, 2017 (pharmacytimes.com)
PTNN, This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- This Week in Managed Care: October 13, 2017 (ajmc.com)
Laura Joszt, assistant managing editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Pharmacist’s ‘deadly’ choices sparked U.S. meningitis outbreak: prosecutors (reuters.com)
A federal prosecutor told jurors...that a Massachusetts pharmacist gambled with patients’ lives by making drugs in unsafe ways that led to a deadly 2012 fungal meningitis outbreak, but a defense lawyer said he was no murderer...Glenn Chin, a former supervisory pharmacist at New England Compounding Center, made drugs in filthy conditions, producing mold-tainted steroids in the process...Those steroids were shipped out to healthcare facilities nationally and then injected into patients, leading to an outbreak that sickened 778 people, including 76 people who died…“Make no mistake, Glenn Chin is not sitting in this court room because he was negligent or careless,”... “He is here because of his deliberate choices.”...Chin directed “massive corner cutting” in...NECC’s so-called clean rooms where the drugs were made, prioritizing production over cleaning and failing to properly test or sterilize drugs.
- Cancer drug study data was falsified, says AstraZeneca (telegraph.co.uk)
An early lab study supporting a cancer drug bought by British drugmaker AstraZeneca was falsified, the company has admitted...AstraZeneca bought a majority stake in US rival Acerta Pharma at the end of 2015 for $4bn on the strength of its novel drug acalabrutinib under development for treating blood cancers and solid tumours. More than 2,000 patients are taking part in more than 25 acalabrutinib clinical trials...Last month Acerta retracted an abstract, published in medical journal Cancer Research in August 2015 – four months before the AstraZeneca investment – purportedly showing the drug was effective in treating solid tumours in mice...AstraZeneca admitted this evidence had been fabricated. It blamed a “former Acerta employee who acted alone to falsify a pre-clinical data set provided through external collaborations”. It confirmed the incident pre-dated its investment and US medicines regulator the Food and Drug Administration had been notified...AstraZeneca added: “It’s important to note that this isolated issue had no impact on the integrity of acalabrutinib data in any clinical trials, and there was no risk to patient health.”...
- Study Finds Few Medicare Limits on Prescription Opioids (ptcommunity.com)
While restrictions are increasing, a third of plans impose none...Medicare plans place few restrictions on the coverage of prescription opioids, despite federal guidelines recommending such restrictions, a new Yale study finds. The research results highlight an untapped opportunity for Medicare formularies to limit opioid prescribing... little is known about opioid coverage and restrictions under Medicare, which often serves as the standard for other insurers...Prescribing restrictions can have an impact...A prior study of a private insurer reported a 15% decrease in opioid prescribing when the insurer implemented restrictions, including prior authorization, quantity limits, and provider–patient agreements...