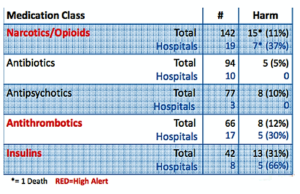
- ISMP Names Top Medication Safety Issues of 2016 (pharmacytimes.com)
It’s crucial for pharmacists to be aware of medications associated with high risk for error and harm to patients, and to look for best ways to implement practices for improving safety and patient care...The Institute for Safe Medication Practices...Compiled data gathered from hospital medication error reports, risk assessments, consumer reports, and FDA collaboration...to name the top medication classes involved in adverse events...the top 5 high-alert medication classes based on data from 2016. Opioids, antithrombotics, and insulins topped the list, followed by antipsychotics and antibiotics...data pegged wrong dosage as the top reason for adverse events in most cases, except in the use of antibiotics, for which wrong drug was the top reason...
- Five things for pharma marketers to know: Wednesday, December 7, 2016 (mmm-online.com)
- President-elect Donald Trump told Time magazine that he “doesn't like what's happened with drug prices” and that he plans to bring down the cost of prescription medications. Time named Trump its Person of the Year on Wednesday. (Time)
- Mylan plans to lay off nearly 10% of its global workforce. A Mylan spokesperson said the job cuts are part of an efficiency strategy. (BioSpace)
- Pfizer was fined $107 million for overcharging the U.K.'s health system for a generic epilepsy drug, phenytoin sodium. The country's antitrust regulator said that Pfizer deliberately unbranded the drug to skirt a competition law. Pfizer said it plans to appeal the ruling. (WSJ)
- Celgene said its understanding of how its multiple myeloma drug Revlimid works in cancer patients is a boon for its R&D efforts in other diseases. The drugmaker currently has three drugs in its pipeline: CC-122 for diffuse large B-cell lymphoma, CC-220 for lupus, and CC-90009 for acute myeloid leukemia. (Bloomberg)
- ICYMI: GlaxoSmithKline said the CEO of ViiV healthcare, Dr. Dominique Limet, will step down and that Deborah Waterhouse, the company's SVP of primary care at GSK U.S. pharmaceuticals, will succeed him. ViiV Healthcare is an HIV specialty company formed in 2009 by GSK and Pfizer.
- This Week in Managed Care: December 2, 2016 (ajmc.com)
Sara Belanger with The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Renown Expands Telehealth To Four Rural Nevada Communities (thisisreno.com)
Renown Health is expanding its video health consultation network to four rural hospitals in Nevada...The videoconferencing service, known as telehealth, allows doctors in Reno to connect with patients in rural areas who may not have access to specialty services, like neurology or pediatrics...Kirk Gillis is the vice president for accountable care with Renown, and he says the need for specialty care in rural areas is critical...“A patient in a rural community, and their primary care provider in that community said, ‘You need to see a specialist.’ The nearest specialist is 200 miles away in Reno, Nevada. They may or may not forego that care, because they may or may not be able to make that trip,” he says...Four hospitals in Nevada have joined the network, including those in Lovelock, Hawthorne, Battle Mountain and Caliente.
- Pharmacy Week in Review: December 9, 2016 (pharmacytimes.com)
Kelly Walsh, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- What Is Bioinformatics and How Does It Relate to Health Care? (pharmacytimes.com)
Tracy Glauser, MD—associate director of Neurology, Cincinnati Children's Hospital Medical Center—discusses the role of bioinformatics in health care.
- Pharmacy Week in Review: December 1, 2016 (pharmacytimes.com)
Ed Cohen, Executive Vice President Pharmacy Advocacy, Pharmacy Times, This weekly video program highlights the latest in pharmacy news, product news, and more.
- Map of all medicines and their mode of action is created (pharmaceutical-journal.com)
Scientists have mapped all 1,578 licensed drugs licensed by the US Food and Drug Administration according to their mechanisms of action to help researchers visualize the ‘uncharted waters’ where they may find future treatments...Scientists at The Institute of Cancer Research in London...extracted data from their own drug database, as well as databases at European Bioinformatics Institute in Cambridge and the University of New Mexico. They matched each drug with prescribing information and data from published scientific papers to build up a picture of how each existing medicine works. The analysis, published in Nature Reviews Drug Discovery (A comprehensive map of molecular drug targets) reveals that there are just 667 unique human proteins targeted by existing approved drugs (or only 3.5% of the estimated 20,000 human proteins), and a further 189 drug targets in pathogenic organisms...This new map of drugs, created through the latest computational analytical technologies, will enhance our ability to use rational, data-driven approaches to identify the most promising future targets and treatment combinations for the next generation of cancer and other diseases...
- Nanowear gains FDA approval for first-of-its-kind wearable: undergarment that tracks cardiac health (drugstorenews.com)
Nanowear, an early stage developer of cloth-based diagnostic monitoring nanosensor technology...announced that it has received FDA Class II 510(k) clearance for its first product, SimplECG, a remote cardiac monitoring undergarment...(it) collects continuous multi-channel ECG, heart rate and respiratory rate data from the garment and transfers it to a web-based portal for review by a physician, by way of a mobile application…it provides accreditation of the company's one-of-a-kind, cloth-based sensor technology as medical-grade...This is the first step and foundation of what we believe to be an extensive array of applications for our nanosensor technology – including numerous other electrical, biometric and biochemical signals that can be measured directly from the skin without conductive gels, adhesives or skin preparation. The market of applications for healthcare alone is a multi-billion-dollar opportunity, but as we look beyond to consumer, industrial, clinical research, military and public sector applications, the addressable market expands exponentially...
- Implementing cloud marketing technology (pharmaphorum.com)
Sanofi Pasteur MSD’s journey to digital has helped sales rep engagement, as well as compliant content creation and distribution...Nearly three years ago, Sanofi Pasteur MSD1 was preparing to launch three new products and decided to take the opportunity to incorporate new, digital channels for better customer engagement… we proposed to reframe our commercial strategy with new technology…The company chose to standardise globally on a cloud-based multichannel CRM solution. Armed with new digital capabilities fully integrated across email, face-to-face, and web, the company’s sales representatives immediately began sending compliant emails directly from the system to customers and personally engaging with healthcare professionals via self-directed, interactive web presentations...The company tripled the expected adoption rates six months ahead of forecasts...To improve the speed of content development and distribution, Sanofi Pasteur MSD took a two-pronged approach...First, it sought to consolidate its agency partners globally, to harmonise content development and increase content reuse across the company...Second, the company looked to streamline content production by adopting a cloud-based commercial content management solution with a critical digital asset management component...The results were transformational, with content production centralised, but with local regions still able to adapt content to meet specific regulatory or cultural needs. And, as it was a cloud-based solution, global agencies now had easy access to promotional assets.