- This Week in Managed Care: November 11, 2016 (ajmc.com)
Laura Joszt, assistant managing editor at The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Zipline raises $25M as it prepares to launch drone delivery medical supply service in U.S. (medcitynews.com)
Zipline, a San Francisco Bay-area company that’s using drones to deliver medical supplies such as blood, (and soon, medication and vaccines) to rural areas and in developing countries, has closed a $25 million Series B round...The funding comes at a time when Zipline is expanding its business in Rwanda and preparing to launch in the U.S...The drone business is planning to launch its drone delivery service in the U.S. next year, as part of a partnership with the White House and the U.S. Federal Aviation Administration...Zipline has been ramping up its medical drone business in Rwanda. The company signed a one-year partnership with Rwanda’s government to deliver blood for transfusions to the western half of the country and plans to start deliveries in the eastern half of Rwanda by the end of the year.
- This Week in Managed Care: November 4, 2016 (ajmc.com)
Justin Gallagher, associate publisher of The American Journal of Managed Care. Welcome to This Week in Managed Care, From the Managed Markets News Network.
- These pricey cholesterol drugs aren’t selling. And that has the biotech industry sweating (statnews.com)
It looked like a surefire way to make billions...A year ago, two new drugs (Repatha and Praluent) that used a novel mechanism to drive down cholesterol levels came on the market, and were promptly crowned as blockbusters in waiting. Analysts estimated sales at more than $3 billion a year...But the two drugs have been commercial flops, in part due to a complicated reimbursement system that has frustrated doctors, confused patients, and left the biotech industry worried about the implications for other high-priced drugs in the pipeline...The failures could send a chill through the still-booming biotech business, which relies on the idea that the risky, expensive process of developing new drugs can one day pay off big... some doctors are hesitant to prescribe them until there’s more information...Another hurdle: Getting insurers to pay for the drugs, which both have list prices of about $14,000 a year...the squabble over cost, access, and availability is unlikely to end any time soon. And the fear among biotech insiders is that the commercial disappointment of PCSK9 therapies will imperil new cardiovascular therapies...
- Pharmacy Week in Review: November 11, 2016 (pharmacytimes.com)
Terri Warholak, PhD, RPh, Associate Professor in the Department of Pharmacy Practice and Science at The University of Arizona; PTNN; This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Buyers clubs for cheaper drugs help fight hepatitis and HIV (reuters.com)
Frustrated by the high price of antiviral drugs, thousands of patients from London to Moscow to Sydney are turning to a new wave of online "buyers clubs" to get cheap generic medicines to cure hepatitis C and protect against HIV infection...While regulators warn that buying drugs online is risky, scientific data presented at a recent medical conference suggest that treatment arranged through buyers club can be just as effective as through conventional channels...The buyers clubs' websites act as middlemen by providing details of trusted online pharmacies and drug manufacturers, exploiting a loophole in World Trade Organization patent rules that allows small-scale imports of medicines for personal use...the advent of today's buyers clubs is just the latest chapter in an ongoing war over drug prices...
- Pharmacy Week in Review: November 4, 2016 (pharmacytimes.com)
Kelly Walsh, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Do links count? FDA to re-examine online drug ads (medcitynews.com)
It’s back to the drawing board for the FDA...After years of slow reaction to pharma marketing online, the agency announced this week a new research initiative to better understand how consumers process short-form posts and ads...the FDA is trying to determine whether links in tweets and Google ads can independently convey all the necessary information about product risk...Under current guidelines, drug companies are required to balance the information they provide in character-space-limited posts. That means for a typical 140-character tweet, at least 70 characters must be dedicated to explaining risks and side effects...Regulatory change could be good news for pharma marketers, who have for a long time sought clarity on what they can and cannot do...
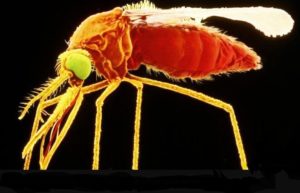
- Walter Reed starts testing Zika vaccine in humans, joining Inovio, NIAID jabs in the clinic (fiercepharma.com)
In a move that illustrates the stepped-up pace of Zika vaccine development, the Walter Reed Army Institute of Research is moving into human testing for its candidate, which uses an inactivated virus to spur immunity to the disease...It's the third Zika-fighting shot to step into the clinic...after Inovio Pharmaceuticals...and the NIH…Walter Reed plans to enroll 75 adults in a Phase I trial of that Zika purified inactivated virus (ZPIV) vaccine...To bolster its Zika effort, the institute has brought in other experts: It partnered with flavivirus vets at Sanofi Pasteur and Brazil’s Fiocruz. The collaborative effort has additional governmental assistance from the National Institute of Allergy and Infectious Diseases, and the Biomedical Advanced Research and Development Authority is contributing funding.
- AI Takes On Drug Safety (fortune.com)
IBM Watson Health tries to do what no pharma company has done: solve the drug-safety puzzle...Big Blue has found yet another business application for its precocious cognitive computing system. IBM Watson Health is collaborating with the biopharmaceutical company Celgene to develop a new platform for evaluating the safety of drugs—both before and after they hit the market—the two companies are announcing this morning. The new offering, "Watson for Patient Safety," will gobble up anonymized medical records, claims data, and millions of electronic submissions to the FDA about potential drug side effects (known as individual case safety reports) to see if it can learn about the hidden dangers of medicines before they become too costly...The problem is one of the toughest in drug development...