- This Week in Managed Care: October 1, 2016 (ajmc.com)
Justin Gallagher, associate publisher of The American Journal of Managed Care. Welcome to This Week in Managed Care, From the Managed Markets News Network.
- This Week in Managed Care: September 24, 2016 (ajmc.com)
Justin Gallagher, associate publisher of The American Journal of Managed Care. Welcome to This Week in Managed Care, From the Managed Markets News Network.
- Allscripts subsidiary 2bPrecise looks to inject genomics into EHR workflow for precision medicine (healthcareitnews.com)
...2bPrecise (subsidiary of Allscripts) is conducting an early adopter program at the National Institutes of Health...The goal is to take clinical and genomic information and make it actionable, structured, machine-readable and machine-learning for physicians. And then take those results and inject the relevant information back into workflows...We focus on the last mile of genomics and cross-collating clinical info with genomics and bring it to the point-of-care...With NIH, we’re putting this to the test and demonstrating the value of genomics in clinical and research activity…People are recognizing genomics is needed, powerful and useful...What’s missing are regulations and reimbursement around this type of data. As healthcare continues to move into this space, I think we’ll see another spike in interest, with a huge level of interest and motivation to try to use genomics more…
- New Big Data Approach Predicts Drug Toxicity in Humans (weill.cornell.edu)
Researchers can now predict the odds of experimental drugs succeeding in clinical trials, thanks to a new data-driven approach developed by Weill Cornell Medicine scientists. The method detects toxic side effects that may disqualify drugs from human use, giving drug developers an early warning before initiating clinical trials, according to a new study...The approach upends conventional wisdom about the criteria on which to evaluate new drugs for their safety. Rather than exclusively examining molecular structure to determine viability, this new computational method combines a host of structural features and features related to how the drug binds to molecules in the body...We looked more broadly at drug molecule features that drug developers thought were unimportant in predicting drug safety in the past. Then we let the data speak for itself…The method, known as PrOCTOR, was inspired by an approach that baseball statisticians adopted to better predict which players would be successful offensively...a strategy known as "Moneyball."...Similarly, researchers developed a computational method that analyzes data from 48 different features of a drug — from molecular weight to details about its target — to determine whether it would be safe for clinical use...this approach could improve the drug discovery pipeline, save money and save lives — but only if more data on toxicity results become available. After all, only 50 percent of clinical trial results are fully reported...if we don't have data, we can't build these models...
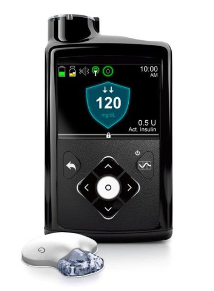
- FDA approves 1st ‘artificial pancreas’ for type 1 diabetes (upi.com)
The Food and Drug Administration...approved the first automated insulin delivery system -- a so-called "artificial pancreas" -- for people with type 1 diabetes...This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin…The device -- Medtronic's MiniMed 670G -- is what's known as a hybrid closed-loop system. That means it monitors blood sugar and then delivers necessary background insulin doses. The device will also shut off when blood sugar levels drop too low...this device isn't yet a fully automated artificial pancreas. People with type 1 diabetes will still need to figure out how many carbohydrates are in their food, and enter that information into the system, the agency noted..
- Survey: 97% plan to use digital health tech in trials over next five years (outsourcing-pharma.com)
According to survey results, published by Validic, more than 60% have used digital health technologies in clinical trials, and more than 97% plan to use such tools more over the next five years…Validic director of marketing told us...Medication adherence has always been a top priority for pharma, which is not surprising given the close correlation between participants’ compliance and the ability to get a drug to market faster and more cost effectively...Given advancements in technology, remotely tracking and monitoring prescription compliance has not only become a reality, but also an increased priority for pharma...As more evidence is available and sponsors continue to realize real-time, objective adherence data enables adaptive trial design and the ability to confidently make adjustments to protocols, we expect to see the interest in adherence technologies continue to rise…mobile applications have been a popular “entry point” for companies looking to being using digital health, “but we’re expecting to see greater use of wearables and sensors in the near-term...the respondents are most interested in reducing trial costs, while also being able to effectively demonstrate a drug’s efficacy in the real world...
- This Week in Managed Care: September 17, 2016 (ajmc.com)
Justin Gallagher, associate publisher of The American Journal of Managed Care. Welcome to This Week in Managed Care, From the Managed Markets News Network.
- Diabetes drugs are badly needed, but rarely make it to market (statnews.com)
Diabetes may be a widespread disease for which millions of people need treatment, but a new analysis finds that developing new medicines has been a risky proposition for drug developers...How so? Here are a few key findings:
- Only 1 in 13 investigational diabetes drugs that entered clinical testing from 1995 to 2007 ultimately received regulatory approval, compared with 1 in 8 for all investigational drugs…
- The likelihood that a diabetes drug entering clinical testing would make it to late-stage testing was about 13 percent...
- the most important challenge for drug makers...continues to be the regulatory approval process, which has grown more demanding in the wake of a controversy in 2008 over the heart risks of a widely used diabetes drug.
- so-called first-in-class approvals for diabetes — which refers to new types of medicines — represented almost 30 percent of all FDA approvals.
- of all new diabetes drugs that were approved by the FDA from 1995 to 2015, 15 percent received a so-called priority review designation...
- The 61 diabetes and other endocrine drugs approved from 1995 to 2015 accounted for 10 percent of all new medicines approved by the FDA during that time...
- Pharmacy Week in Review: September 23, 2016 (pharmacytimes.com)
Kelly Walsh, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Pharmacy Week in Review: September 16, 2016 (pharmacytimes.com)
Kelly Walsh, PTNN. This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.