- Pharmacy Week in Review: June 30, 2016 (pharmacytimes.com)
Mike Glaicar, Business Development: Pharmacy Times...(PTNN) This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
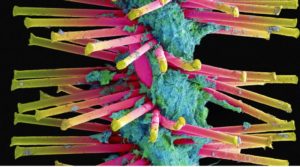
- Slimy clumps of bacteria kill thousands. Scientists are fighting back (statnews.com)
It’s a battle that seems ripped from a sci-fi film: Scientists are racing to develop new weapons to destroy the slimy colonies of bacteria, known as biofilms, that cause tens of thousands of deaths across the US each year...Biofilms are the leading cause of infections acquired in hospitals. They grow on medical devices such as heart valves, pacemakers, and catheters. They take root inside wounds, pulsing and rippling as they spread...Encased in gooey protective sheaths, biofilms are exceptionally hard to stop. Many are impervious to antibiotics. They also cost the health care system billions each year, as patients often require surgery to remove and replace contaminated implants...researchers and biotech startups are testing new methods of attack, from coating medical devices with spiky coverings to blasting bacteria with electrical fields to interrupting the chemicals that cells inside biofilm colonies use to send messages to each other...researchers at Ohio State announced they’d invented a way to coat the surfaces of medical devices with Y-shaped nanoparticles of quartz in a bid to block biofilms from latching on tight...
- Here’s a look at the battle:
- What are biofilms?
- Is this a new menace?
- Why are biofilms so hard to kill?
- So, what’s being done?
- Pharmacy Week in Review: June 17, 2016 (pharmacytimes.com)
Mike Glaicar, Business Development: Pharmacy Times...(PTNN) This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- FDA seeks suspension of 4,402 illegal prescription drug websites (reuters.com)
The U.S. Food and Drug Administration said...along with international authorities, has formally sought to suspend 4,402 websites that illegally sell potentially dangerous, counterfeit or unapproved prescription drugs to U.S. consumers...The move is part of a global effort being led by the INTERPOL...to identify the makers and distributors of illegal prescription drugs...the FDA said it has also issued warning letters to operators of 53 websites that illegally sell unapproved and misbranded prescription drug products to U.S. consumers...Preliminary findings...showed U.S. consumers had purchased certain unapproved drug products from abroad to treat depression, narcolepsy, high cholesterol, glaucoma, and asthma, among other conditions...
- Pfizer building modular biologics plant in China (fiercepharma.com)
Pfizer will build a biologics plant in China, where it will make biosimilars for the Chinese market but also for sale throughout the world. The New York drugmaker has turned to GE for construction of the facility, which has developed a modular construction process that will cut the cost and allow the plant to be operational in about 18 months, instead of three years...The $350 million facility, which it is building at the Hangzhou Economic Development Area in China, will be Pfizer’s third biologics production facility and its first in Asia. In addition to manufacturing, the facility will house Pfizer China’s Biosimilars and Biologics Quality, Technical Service, Logistics and Engineering divisions, and serve as a site for process development and clinical supplies. It will have about 150 employees when it is complete in 2018…The new center will be built using GE’s single-use technology…likened assembly to working with LEGO blocks...
- This Week in Managed Care: June 25, 2016 (ajmc.com)
Justin Gallagher, associate publisher of The American Journal of Managed Care. Welcome to This Week in Managed Care from the Managed Markets News Network
- Can a Bunch of Doctors Keep an $8 Billion Secret? Not on Twitter (bloomberg.com)
In New Orleans...a major medical organization attempted a feat perhaps as hard as treating the disease doctors were there to discuss. They asked a packed convention hall of attendees not to tweet the confidential, market-moving data they had flown in to see...It didn't work...In an unusual arrangement, the American Diabetes Association let hundreds, if not thousands, of in-person attendees see new data on Novo Nordisk A/S・s blockbuster diabetes treatment Victoza (liraglutide) more than an hour before its official release to the public and the markets. That's atypical for such sensitive data, which are usually shared only with journalists and researchers who have agreed to abide by strict terms, under threat of losing future access...After warning attendees not to share the information they were about to post...Within minutes, some Twitter accounts were posting pictures of the charts, including key slides that showed the drug's success in reducing deaths. And as fast as the posts went up, the medical society's communications team issued online pleas for them to stop...Novo's shares fell 5.6 percent to 343 kroner, for their biggest one-day drop since February...You can't embargo something that is being discussed publicly...Why are they trying to control the flow of information, especially in this case where the results could influence public health and the markets? Hopefully other organizations won't take this as a signal they can do the same thing...
- Magnetic blood clot dissolver could be 4,000 times more efficient than enzyme treatment (fiercepharma.com)
Researchers at ITMO University in St. Petersburg, Russia, have developed a magnetically controlled treatment designed to dissolve blood clots. The method looks to be a promising solution to some of the complications associated with enzyme-based thrombolytic drugs...To make the targeted drug, the scientists combined the mineral magnetite with the enzyme urokinase, commonly used as a thrombolytic agent. The nanosized particles can then be localized around a blood clot using an external magnetic field...The combination demonstrated up to 4,000 times more efficiency than the enzyme-based drugs alone...Now we are using a sledgehammer to crack a nut… In order to change the situation, we decided to develop a method of targeted drug delivery that would allow us to considerably reduce the dosage and ensure that the whole therapeutic effect is focused on the clot...
- Pharmacy Week in Review: June 24, 2016 (pharmacytimes.com)
Mike Glaicar, Business Development: Pharmacy Times...(PTNN) This weekly video program provides our readers with an in-depth review of the latest news, product approvals, FDA rulings and more.
- Pharmacy Week in Review: June 10, 2016 (pharmacytimes.com)
Brian Haug, President of Pharmacy and Managed Markets, Pharmacy Times (PTNN) This weekly video program highlights the latest in pharmacy news, product news, and more.