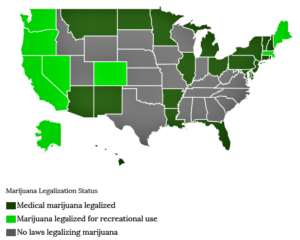
- A New Test of Pot’s Potential to Replace Painkillers (theatlantic.com)
Emily Lindley’s stash of marijuana is going to be very, very secure...Lindley, a neurobiologist, is about to begin the first study ever to directly compare cannabis with an opioid painkiller for treating people with chronic pain. She got a grant for this research two years ago, but it has taken that much time to meet all the requirements for working with a drug the federal government still considers highly dangerous...The current status of medical marijuana research is rife with irony. As states have liberalized marijuana laws, they’ve created new opportunities: Lindley’s grant is part of $9 million Colorado awarded for medical research in 2014, using tax money from marijuana sales. But since pot remains illegal at the federal level, researchers have to jump through regulatory hoops—lots of them—to do legitimate research...Physicians have commented for more than a century on the potential for cannabis to substitute for opioid drugs, and several recent studies seem to bolster this hypothesis...The idea has not been tested with rigorous clinical trials, however. Such trials are expensive, and they’re normally paid for by a pharmaceutical company hoping to bring a new drug to market. Because a plant that exists and reproduces in nature can’t be patented, cannabis offers few opportunities for patents (and thus profits), which makes it look like a loser to most companies...Lindley is eager to get on with her study...Given that so many people are already self-treating with marijuana, Lindley says, it’s important to know whether there are actually therapeutic effects. “I’m not a proponent one way or another,” she says. “I think we need to do the research.”
- New Big Data Approach Predicts Drug Toxicity in Humans (weill.cornell.edu)
Researchers can now predict the odds of experimental drugs succeeding in clinical trials, thanks to a new data-driven approach developed by Weill Cornell Medicine scientists. The method detects toxic side effects that may disqualify drugs from human use, giving drug developers an early warning before initiating clinical trials, according to a new study...The approach upends conventional wisdom about the criteria on which to evaluate new drugs for their safety. Rather than exclusively examining molecular structure to determine viability, this new computational method combines a host of structural features and features related to how the drug binds to molecules in the body...We looked more broadly at drug molecule features that drug developers thought were unimportant in predicting drug safety in the past. Then we let the data speak for itself…The method, known as PrOCTOR, was inspired by an approach that baseball statisticians adopted to better predict which players would be successful offensively...a strategy known as "Moneyball."...Similarly, researchers developed a computational method that analyzes data from 48 different features of a drug — from molecular weight to details about its target — to determine whether it would be safe for clinical use...this approach could improve the drug discovery pipeline, save money and save lives — but only if more data on toxicity results become available. After all, only 50 percent of clinical trial results are fully reported...if we don't have data, we can't build these models...
- Behind rosy predictions, life sciences execs reveal unsettling concerns (statnews.com)
If you ask a life sciences chief executive to gaze into a crystal ball, he or she will tell you there is good reason to be optimistic about the future. Or so a new survey would have us believe....All 38 executives who participated in the survey reported that they are confident about what lies ahead; 79 percent are convinced their products will remain relevant for the next few years; and 97 percent are certain that they are staying on top of coming trends...What might explain such a bullish view of the world? Well, chief executives...by their very nature, tend to be optimistic people and the industry is coming to terms with its challenges...This may be a case, however, of seeing the world through the proverbial rose-colored glasses...Why? At the same time these chief executives are so upbeat, a whopping 89 percent also confessed they are concerned they will not be able to increase market share. And 74 percent expect top-line growth of between just 2 percent and 4 percent over the next three years...We wonder if their investors know how they feel about such prospects.
- GSK and Google launch bioelectronics venture (pmlive.com)Q&A: Glaxo exec says bioelectronics is ‘not science fiction’ (statnews.com)
GlaxoSmithKline has joined forces with Google's Verily Life Sciences to establish a new company dedicated to the development of bioelectronic medicines...Galvani Bioelectronics - named for the 18th century Italian bioelectricity pioneer - will combine GSK's drug discovery and development expertise in disease biology with Verily's expertise in highly miniaturised technologies, including data analytics and software development for clinical application...Together, we can rapidly accelerate the pace of progress in this exciting field, to develop medicines that truly speak the electrical language of the body...Bioelectronic medicine is concerned with the electrical signals firing between the body's nervous system and organs, working to regulate the faulty nerve impulses that occur in many illnesses.
- Walter Reed starts testing Zika vaccine in humans, joining Inovio, NIAID jabs in the clinic (fiercepharma.com)
In a move that illustrates the stepped-up pace of Zika vaccine development, the Walter Reed Army Institute of Research is moving into human testing for its candidate, which uses an inactivated virus to spur immunity to the disease...It's the third Zika-fighting shot to step into the clinic...after Inovio Pharmaceuticals...and the NIH…Walter Reed plans to enroll 75 adults in a Phase I trial of that Zika purified inactivated virus (ZPIV) vaccine...To bolster its Zika effort, the institute has brought in other experts: It partnered with flavivirus vets at Sanofi Pasteur and Brazil’s Fiocruz. The collaborative effort has additional governmental assistance from the National Institute of Allergy and Infectious Diseases, and the Biomedical Advanced Research and Development Authority is contributing funding.
- This is how 3-D bioprinting companies are transforming drug development (medcitynews.com)
... cells generally like having company, forming communities with other cell types to make complete tissues. However, quite often, researchers isolate cells from their 3-D environments, creating 2-D models that don’t always replicate complex biology...But now, an entire industry is emerging to recapitulate various tissues more realistically. 3-D bioprinting has the capacity to layer different cell types, creating more biologically accurate liver, kidney, skin and even tumor tissues. The hope is that more lifelike tissues will produce better scientific results...Organovo is the leader in 3-D bioprinting...The...company has been selling 3-D liver tissue to pharma companies for about 18 months and is about to expand into kidney tissue. The goal is to support drug development by using these 3-D models to gauge toxicity...Kidney and liver toxicology studies are required for every product before it goes into humans....The high costs of clinical trials could eventually make this a go-to technology for pharma and biotech, providing better data to spot toxicity early and avoid costly mistakes…
- Exploiting body’s fat absorption pathways may improve drug efficacy (upi.com)Glyceride-Mimetic Prodrugs Incorporating Self-Immolative Spacers Promote Lymphatic Transport, Avoid First-Pass Metabolism, and Enhance Oral Bioavailability (abstract) (onlinelibrary.wiley.com)
Many medications are broken down before making it to the bloodstream, preventing their arrival at the site of infection, but researchers think they've found a way to improve drug delivery by bypassing certain bodily processes...Researchers...have created a method of delivering drugs using the lymphatic system in order to bypass the liver and create a route directly to the bloodstream, increasing the amount of a substance making it to target areas...The advantage of our system is that drugs are shielded from degradation in the liver but are ultimately released when they reach their site of action, ensuring that the drug given to the patient goes where it is supposed to...researchers created a technology to modify drugs so they mimic dietary lipids, which are absorbed into the lymph system -- unlike other nutrients...No matter how good the drug is, it needs to be absorbed [into the bloodstream] and to avoid this first pass metabolism in order to get to the general circulation where it acts...
- Diabetes drugs are badly needed, but rarely make it to market (statnews.com)
Diabetes may be a widespread disease for which millions of people need treatment, but a new analysis finds that developing new medicines has been a risky proposition for drug developers...How so? Here are a few key findings:
- Only 1 in 13 investigational diabetes drugs that entered clinical testing from 1995 to 2007 ultimately received regulatory approval, compared with 1 in 8 for all investigational drugs…
- The likelihood that a diabetes drug entering clinical testing would make it to late-stage testing was about 13 percent...
- the most important challenge for drug makers...continues to be the regulatory approval process, which has grown more demanding in the wake of a controversy in 2008 over the heart risks of a widely used diabetes drug.
- so-called first-in-class approvals for diabetes — which refers to new types of medicines — represented almost 30 percent of all FDA approvals.
- of all new diabetes drugs that were approved by the FDA from 1995 to 2015, 15 percent received a so-called priority review designation...
- The 61 diabetes and other endocrine drugs approved from 1995 to 2015 accounted for 10 percent of all new medicines approved by the FDA during that time...
- Retail Pharmacist Salary Growth Stalls, while Hospital Pharmacists’ Salaries Rise (drugchannels.net)
There’s some bad news in our latest exclusive annual analysis of pharmacist salaries, based on the Bureau of Labor Statistics’ recently released Occupational Employment Statistics...In 2015, the average gross base salary for a pharmacist at a retail, mail, and specialty pharmacy was $119,517—up a paltry 0.1% from 2014. Retail employment grew slowly, with slightly fewer pharmacists working at drugstores and mass merchants...Meanwhile, employment at mail pharmacies and hospitals grew. Pharmacists who work at hospitals also got some good paycheck news: Their salaries rose by 1.6%. The share of pharmacists who work at hospitals grew, too...The pharmacy industry’s ongoing shift from traditional to specialty drugs is altering long-standing pharmacist employment patterns…
Here are my observations about 2015 trends:
- Mass merchants still ahead.
- Mail employment rose sharply.
- Hospital employment keeps growing.
- Pharmacist salaries exceeded those of other healthcare workers, but growth lagged in 2015.
- Prescription drug abuse epidemic extends beyond the United States (rti.org)
There is a high rate of prescription pain reliever abuse in Europe, largely accounted by opioids, according to the first comparative study of prescription drug abuse in the European Union, which was conducted by researchers at RTI International....The study investigated nonmedical prescription drug use in five European countries – Denmark, Germany, Spain, Sweden and the United Kingdom...For certain classes of medications, like opioids, we found a significant rate of prescription pain reliever abuse in the EU...While the lifetime rates were not as high as in the U.S. – 20 percent for those aged 12 years and over, compared to between 7 percent and 13 percent in the EU – the past-year rates were only slightly lower. This suggests that the EU may be catching up to the United States for some substances...Previously, it was thought that the prescription drug epidemic was limited to the United States...but this study shows that the epidemic extends well beyond the U.S...Identification of the scope and prevalence of nonmedical prescription drug use in the EU is an important first step in building a worldwide system that can be used to monitor trends, track risk and protective factors and to develop targeted interventions aimed at reducing the risk of nonmedical prescription drug use...