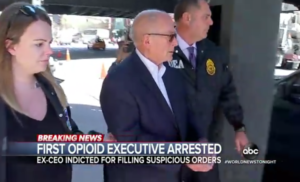
- Feds charge Rochester Drug Cooperative and CEO in first criminal case over opioids (abcnews.go.com)
Federal prosecutors charged drug distributor Rochester Drug Cooperative and its former CEO with drug trafficking charges...the first criminal charges for a pharmaceutical company and executives in the nation's ongoing opioid crisis... This prosecution is the first of its kind: executives of a pharmaceutical distributor and the distributor itself have been charged with drug trafficking...The U.S. Attorney's Office for the Southern District of New York charged Rochester Drug Cooperative...with "knowingly and intentionally" violating federal narcotics laws "by distributing dangerous, highly addictive opioids to pharmacy customers that it knew were being sold and used illicitly,"...RDC was also charged with failing to properly report thousands of suspicious orders of oxycodone, fentanyl and other controlled substances to the Drug Enforcement Agency...READ MORE
- U.S. Supreme Court rejects Allergan bid to use tribe to shield drug patents (reuters.com)
The U.S. Supreme Court...cast aside pharmaceutical company Allergan Plc’s unorthodox bid to shield patents from a federal administrative court’s review by transferring them to a Native American tribe...The justices left in place a lower court ruling upholding the authority of a U.S. Patent and Trademark Office tribunal to decide the validity of patents covering Allergan’s dry eye drug Restasis, refusing to hear the company’s appeal. Allergan had argued that the tribe’s sovereign status under federal law made the patents immune from administrative review by the agency...Allergan, which has its headquarters in Dublin, in September 2017 transferred the patents to New York’s Saint Regis Mohawk Tribe, which took legal ownership of the patents and then licensed them back to Allergan in exchange for ongoing payments...Allergan said it was protecting itself from the patent court, which it called a flawed and biased forum...READ MORE
- Swiss drugmaker Novartis must face doctor kickback suit, U.S. judge rules (reuters.com)Did Novartis hand out kickbacks or host educational dinners? A jury may decide (fiercepharma.com)
Novartis AG must face a U.S. government lawsuit accusing it of paying millions of dollars in kickbacks to doctors so they would prescribe its drugs, after a federal judge ruled in a decision...that the government had offered evidence of a “company-wide kickback scheme.”...U.S. District Judge Paul Gardephe in Manhattan also rejected the Swiss drugmaker’s bid to keep key government evidence out of the case, and ruled that the government does not have to prove a direct “quid pro quo” agreement between Novartis and doctors for the company to be liable...READ MORE
- CBD is booming. But US farmers struggle to keep up with demand for industrial hemp (cnbc.com)
Congress legalized industrial hemp in December. With it, they also legalized hemp-derived CBD, short for cannabidiol, a cannabis compound that supposedly delivers the calming effects of marijuana without the high from THC...Last year, retail sales of CBD consumer products in the U.S. were estimated at between $600 million and $2 billion, according to investment research firm Cowen. The bank conservatively forecasts sales to reach $16 billion by 2025, with health and wellness products leading the way and food, beverage, beauty and vapor to also play a role.
- From seed to CBD - The current supply chain — from plants, to extraction, to labs — is riddled with issues...
- More religion than science
- Incredibly expensive
- A ‘green rush’
- Vastly different results
- Wild West...READ MORE
- Critics take aim at plan to allow prescription drug imports from Canada (pressherald.com)
Pharmacists and some health experts are opposing a proposal to permit the bulk importation of drugs from Canada to Maine, arguing that it could result in unsafe drugs being brought into the state and have unintended consequences, such as causing drug shortages in Canada...Proponents of Senate President Troy Jackson’s bill say those fears are unfounded, and believe it could be one of several proposals that would help to rein in prescription drug prices in Maine...If approved, the state would designate a wholesale purchaser of drugs from Canadian wholesalers...READ MORE
- Exclusive: Pain-care specialist agrees to testify against Purdue, other drug makers – court documents (reuters.com)
A physician ally of Purdue Pharma LP...has agreed to testify against the OxyContin maker and other drug companies, newly disclosed court records show...Dr. Russell Portenoy, a professor at the Albert Einstein College of Medicine, was an early advocate for the use of opioids for chronic pain...He also was named as a defendant in some of the lawsuits filed by cities, counties and states seeking to hold opioid makers - including Endo and Mallinckrodt Plc...But Portenoy...struck a deal with the plaintiffs to serve as a cooperating witness, the records show. In exchange for his dismissal from the suits, Portenoy provided the plaintiffs with documentation of opioid makers’ payments to him over the years, as well as a 36-page declaration that lays out what he would say on the witness stand...READ MORE
- Purdue Pharma agrees to $270 million settlement in Oklahoma opioid case (reuters.com)
OxyContin maker Purdue Pharma LP...reached a $270 million settlement to resolve a lawsuit brought by the state of Oklahoma accusing the drugmaker of fueling an opioid abuse epidemic...The settlement unveiled by Oklahoma Attorney General Mike Hunter...was the first to result from a wave of lawsuits accusing Purdue of deceptively marketing painkillers, helping create a deadly crisis sweeping the United States...Hunter alleged Purdue, Johnson & Johnson and Teva Pharmaceutical Industries Ltd engaged in deceptive marketing that downplayed the addiction risk from opioids while overstating their benefits, contributing to the epidemic...READ MORE
- U.S. charges pummel drugmaker Indivior, hurt Reckitt (reuters.com)
Indivior Plc lost nearly three-quarters of its stock market value...and former parent Reckitt Benckiser also fell after the U.S. Justice Department accused the British drugmaker of illegally boosting prescriptions for its blockbuster opioid addiction treatment Suboxone...An indictment...alleged Indivior made billions of dollars by deceiving doctors and healthcare benefit programs into believing the film version of Suboxone was safer and less susceptible to abuse than similar drugs...The indictment charged Indivior and its subsidiary Indivior Inc with conspiracy, health care fraud, mail fraud and wire fraud. The U.S. government said it would seek to have it forfeit at least $3 billion...READ MORE
- Pharma spends big as Massachusetts lawmakers review drug-pricing bills (fiercepharma.com)
State legislators in Massachusetts are preparing to review a slew of bills aimed at state-level drug pricing, and the pharmaceutical industry is opening its checkbook to fight back...The state’s Joint Committee on Health Care Financing on April 11 will examine 21 bills targeting price transparency and affordability for patients. State Sen. Cindy Friedman...will take a hard look at measures to open up drugmakers' pricing secrets and lower sticker prices for patients...But pharmaceutical companies aren’t taking that challenge sitting down...the Pharmaceutical Research Manufacturers of America, told FiercePharma that the state’s push to cap prices would keep patients from getting the drugs they need...“We oppose government price-setting proposals in the states,” spokeswoman Priscilla VanderVeer said. “Government price controls limit access to medicines for patients and they stifle new innovation.”...READ MORE
- Eli Lilly sheds light on confidential drug pricing, discloses charges for popular diabetes medicine Humalog (cnbc.com)
...Eli Lilly pulled the curtain back on the confidential pricing structure for one of its blockbuster drugs...disclosing for the first time what it charges wholesalers versus what many patients typically pay...The company’s list prices for its popular insulin injection Humalog, versus what most patients are charged after insurance company rebates and other discounts, highlight the disparity in prices between uninsured and insured patients. The move is also a pre-emptive one as the Trump administration and Congress pressure drugmakers for more transparency and to lower drug costs...The “net price ” patients actually pay for Eli Lilly’s insulin fell by 8.1 percent to $135 a patient per month in 2018 from $147 in 2014...The net price is the total paid after factoring in rebates and discounts. The insulin’s average list price before the discounts rose 51.9 percent to $594 per patient each month...The Trump administration earlier this year proposed a rule to end the industry-wide system of rebates, a change that Lilly and other pharmaceutical companies welcomed...READ MORE