- Valeant faces new trouble as dermatologists sour on its drugs (statnews.com)

FILE PHOTO. The head offices of Valeant Pharmaceutical is pictured in Montreal on Monday May 27, 2013. THE CANADIAN PRESS/Ryan Remiorz
Yet another sign of trouble for Valeant Pharmaceuticals has emerged. The beleaguered drug maker already faces congressional hearings into its pricing practices. Now, one of its bread-and-butter product lines may not ring the register as often as before…Fewer dermatologists are prescribing Valeant medicines…The change constitutes more fallout from a scandal involving the drug maker’s relationship with a mail-order pharmacy known as Philidor Rx Services. Valeant reportedly hid its ties to the pharmacy in order to inappropriately boost prescriptions and insurance reimbursements...68 percent are writing fewer prescriptions for Valeant’s dermatology products and a similar percentage expect they will not prescribe Valeant drugs in coming months.
- California proposes new single-drug method for executions (latimes.com)
California unveiled a new method for executing condemned prisoners Friday, proposing a single-drug lethal injection protocol that could restart capital punishment…The proposal came as a result of a lawsuit filed against the state by crime victims…Executions are not likely to resume immediately…The new protocol would require the injection drug to be selected on a "case-by-case basis, taking into account changing factors such as the availability of a supply of chemical." The state would have the option of using one of four barbiturates: amobarbital, pentobarbital, secobarbital and thiopental…Executions around the country have declined in recent years as prisons have been unable to obtain lethal injection drugs…Manufacturers, pressed by death penalty opponents, have refused to sell the anesthetics to prisons. Compounding pharmacies are an alternative, but even they would be vulnerable to boycotts if their identities were disclosed…
- Pharmacists Bringing Patient Care Services to Capitol Hill – October 28 (pharmacist.com)
As part of its advocacy and community outreach efforts during American Pharmacists Month, the American Pharmacists Association is partnering with the co-chairs of the House Community Pharmacy Caucus, the National Association of Chain Drug Stores, the National Community Pharmacists Association, the American Society of Health-System Pharmacists, and Walgreens to host the 3rd Annual Capitol Hill Health Fair on October 28. Flu shots and health screenings, including bone density, glucose, cholesterol, blood pressure, and body composition will be available to members of Congress, staff, and the general public in the Rayburn Foyer in the Rayburn House Office Building from 10:00 am to 2:00 pm.
Pharmacists from Walgreens and student pharmacists from:
- University of Maryland Eastern Shore School of Pharmacy
- University of Maryland School of Pharmacy
- Notre Dame of Maryland University School of Pharmacy
- Howard University College of Pharmacy
- Shenandoah University Bernard J. Dunn School of Pharmacy
- Virginia Commonwealth University School of Pharmacy
- Pacira sues for free speech; FDA pulls off-label warning letter. What gives? (fiercepharmamarketing.com)
On second thought, Pacira Pharmaceuticals, the FDA might just take it back...the agency has unpublished a warning letter to the company, issued last September, that took issue with the company's marketing practices…the FDA warning letter--now taken down from the agency website--Pacira had claimed that Exparel (bupivacaine), its pain drug and lead product, could work for up to three days at a time, though it's only approved for 24-hour pain relief… It would seem like a good old-fashioned pharma-government tussle over what companies can and cannot say about their products. But the argument has changed significantly, thanks to a series of court rulings that could open the door for drugmakers to market their products in previously unacceptable--even previously illegal--ways...
- 5 Tips to Keep Patients Loyal to Your Pharmacy (pharmacytimes.com)What Makes Patients Loyal to a Pharmacy? (pharmacytimes.com)Pharmacy Mistakes That Can Disrupt Patient Loyalty (pharmacytimes.com)
Once a pharmacy has attracted a solid base of patients, the next step is keeping them coming…pharmacies can maintain a loyal community of patients…patients are on autopilot, so their habits may lead them to the same pharmacy every time. However, other patients may have medications filled at multiple pharmacies, and some of them may be looking for a home base…Here are 5 tips to keep patients loyal to your pharmacy:
- Let patients know what you do, and tell your story
- Implement an adherence program
- Investigate your pharmacy’s challenges
- Know your market
- Find your niche
- Benefits of E-Prescribing Over Traditional Prescriptions (pharmacytimes.com)
Ken Whittemore Jr, Surescript's senior vice president of professional and regulatory affairs, discusses some of the ways in which e-prescribing trumps traditional prescriptions. (video)
- FDA Makes Recommendation to PCAC on Additions to Bulk Drugs (iacprx.org)
Next week, on October 27 and 28, the FDA Pharmacy Compounding Advisory Committee is reviewing ten (nine) active pharmaceutical ingredients to be included on the list of approved bulk drugs that traditional compounders can use in preparing prescriptions. As required within 503A, a bulk ingredient must have an applicable USP monograph, be a component of an FDA approved drug, or be added to an official list authorized by the Secretary of Health and Human Services. The ten (nine) drugs to be reviewed were nominated by stakeholders…in response to formal requests issued by the agency…FDA has released documents for the PCAC that show their recommendations on each of the nominated drugs. (each item, FDA does not recommend addition to the approved bulk ingredient list)
- Methylsulfonylmethane
- Curcumin
- Germanium Sesquioxide
- Rubidium Chloride
- Deoxy-D-Glucose
- Alanyl–L-Glutamine
- Glycyrrhizin
- Glutaraldehyde
- Domperidone
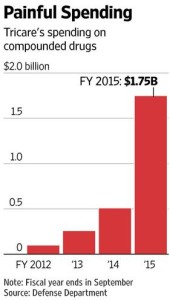
- U.S. Targets Pharmacies Over Soaring Claims to Military Health Program (wsj.com)
Settlements sought for prescriptions written in cases where doctors never met patients…Federal prosecutors in at least four states are mounting investigations into what they describe as widespread fraud by compounding pharmacies in claims to the health-insurance program that covers 9.5 million U.S. military members and their families…In the latest move, four Florida pharmacies last month agreed to pay $12.8 million combined to settle civil allegations that they falsely billed the insurance program Tricare for expensive pharmaceutical creams and gels to treat pain, scars and other ailments…Two of the compounding pharmacies...employed salespeople who paid doctors to write prescriptions to Tricare beneficiaries…In some cases, doctors would conduct telephone consultations with beneficiaries and then write them prescriptions, despite having not met with the beneficiaries in person…Those prescriptions were illegitimate because they weren’t based on genuine doctor-patient relationships, a violation of the federal False Claims Act...
- Pharmacy Track-and-Trace Compliance Deadline Again Delayed by FDA (pharmacytimes.com)Are you ready for the Drug Supply Chain Security Act? (fda.gov)
FDA has once again delayed its enforcement of product tracing requirements for pharmacies under the Drug Supply Chain Security Act...Although these track-and-trace requirements took effect on July 1, 2015, the FDA said it would not enforce the product tracing obligations for pharmacies until November 1, 2015, which it has now extended to March (March 1,2016)…FDA granted this latest extension because “some dispensers—primarily smaller, independent pharmacies and health systems—have expressed that they need additional time,” …In the meantime, pharmacies that do not capture and maintain product tracing information, or accept prescription drugs without product tracing information prior to or during a transaction, will not be penalized…this compliance policy does not extend to other requirements for dispensers under the DSCSA, which include verification related to suspect and illegitimate product (including quarantine, investigation, notification, and record keeping) and engaging in transactions only with authorized trading partners.
- Compounder targets Turing’s now-pricey Daraprim with $1-per-pill alternative (fiercepharma.com)
..Imprimis says it will offer a compounded drug that includes Daraprim's active ingredient--pyrimethamine--in capsules starting at $99 for a 100-count bottle. The company is also starting a program to work with payers, pharmacy benefits managers and purchasing groups to offer patient-specific formulations "at prices that ensure accessibility."…The compounded drug isn't an exact copy; it also includes the ingredient leucovorin, which…helps to combat pyrimethamine's negative effects on bone marrow…After the meningitis outbreak linked to the New England Compounding Center, new regulations tightened up on distribution of compounded drugs, which aren't specifically approved by the FDA. Compounded meds can only be dispensed on specific prescriptions for specific patients, rather than distributed in bulk as FDA-approved products are...