- FDA knocks Tennessee compounder for raft of cleanliness issues (fiercepharma.com)
FDA investigators cited Tennessee compounder Surgery Pharmacy Services for a host of cleanliness issues at its Chattanooga plant, including workers' failure to change gloves after removing trash bins and to sanitize supplies carried between clean rooms...Inspectors further knocked Surgery for using a laminate work surface in one of its clean rooms that was "worn, stained, and chipped," as well as using a nonsterile disinfectant to clean one of the rooms...READ MORE
- FIP calls for focus on pharmacy remuneration (fip.org)
Pharmacist remuneration models in ever-evolving healthcare settings must be examined if global health is to be supported...“A common concern among pharmacy associations worldwide is the long-term financial viability of professional services delivered by pharmacists. These concerns are first and foremost triggered by the impacts of continued price and margin cuts in dispensing of medicines and the non-allocation of funding in many settings for extended professional services and social care,”...“A successful remuneration model is one that promotes sustainable delivery of professional services. These should be integrated into broader health system strategies and, therefore, funding plans...READ MORE
- APC Launches Shortage Drug Source to Connect Hospitals with Compounders (drugtopics.com)
The Alliance for Pharmacy Compounding announced the launch of its free resource, which serves as a liaison between hospitals with 503B outsourcing facilities, or 503A sterile compounding pharmacies, that can supply treatments currently in shortage due to the COVID-19 pandemic...APC’s Compounders’ Shortage Drug Source for Hospitals mimics its earlier online bulletin board...The news release provided information to hospitals interested in utilizing the resource:
• FDA-registered 503B outsourcing facilities and qualified, board of pharmacy-approved 503A sterile compounders may provide the resource with information about their available supplies. Only those shortage drugs listed by FDA in appendices to its recent temporary guidance document for 503Bs and 503As may be listed.
• Data submitted by outsourcing facilities and 503A pharmacies will post this information for hospitals to access.
• If hospitals are unable to source the needed drug(s) from a 503B, they may access a secondary page listing information submitted by 503A pharmacies about shortage drugs they can prepare...
• APC has asked the American Hospital Association and the American Society of Health System Pharmacists to make its hospital members aware of the resource...READ MORE
- Local pharmacies overwhelmingly need coronavirus small business aid as reimbursements and cash flow decline (chaindrugreview.com)
Nearly 90% of community pharmacies will apply for small business federal aid under the CARES Act to help them get through the coronavirus storm, according to a new survey...by the National Community Pharmacists Association...“Pharmacies are ‘essential businesses’ staying open during the COVID-19 pandemic to keep serving their communities, but many are on the brink at the very time they are needed most,” said NCPA CEO B. Douglas Hoey. “In the 18 months before this crisis, the number of pharmacies had shrunk by over 2,000, mostly due to low reimbursement from pharmacy benefit managers...As the pandemic strains neighborhood pharmacies, nearly half of pharmacy owners rank the overall financial health of their business as somewhat poor or very poor, according to the survey...READ MORE
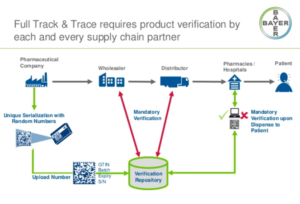
- Pharmacies must comply with certain track-and-trace requirements by November 27, FDA says (pharmacist.com)
Despite APhA’s concerns that compliance with certain Drug Supply Chain Security Act requirements undermines pandemic response efforts, FDA has confirmed that “dispensers,” including pharmacies, must meet a requirement that goes into effect on November 27, 2020. APhA and pharmacy partners had requested that FDA delay enforcement of certain portions of the DSCSA—also known as “track and trace”—mandate...READ MORE
- FDA blasts California compounding pharmacy for facility ‘contaminated with filth’ (fiercepharma.com)
Auro Pharmacy has had a number of run-ins with FDA investigators...The FDA has had a long and troubled history with compounding pharmacies...sometimes those facilities bring it on themselves...Auro Pharmacies operated a veritable house of horrors at its...outsourcing facility, with ants in the sterile production areas and visibly dirty work surfaces, FDA investigators found during an August 2018 inspection...Those poor conditions could have produced supposedly sterile drugs that were "contaminated with filth,"...The FDA knocked Auro with a 10-observation Form 483 in August, 2018 that led to a voluntary recall of all the pharmacy's affected drugs and a stoppage to all sterile production...Even worse for Auro, the FDA followed up its August 2018 look-in with another round of inspections in September of last year that turned up most of the same sanitary issues, including "filth" on the end of hood-cleaning wands, and cracked and scratched production hoods...That inspection lead to a separate 11-observation Form 483 sent in October...READ MORE
- CDC Guidance for Community Pharmacies During COVID-19 (drugtopics.com)Guidance for Pharmacies - Guidance for Pharmacists and Pharmacy Technicians in Community Pharmacies during the COVID-19 Response (cdc.gov)Using Personal Protective Equipment (PPE) (cdc.gov)Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings (cdc.gov)
All pharmacy staff—along with everyone entering the pharmacy—should wear face masks, according to new guidance from the CDC...The CDC’s “Guidance for Pharmacists” also says that pharmacies should postpone and reschedule some routine preventive services, such as adult immunizations, since they require face-to-face interaction...This guidance applies to all pharmacy staff to minimize their risk of exposure to the virus and reduce the risk for customers during the COVID-19 pandemic...According to the guidance, pharmacies should:
Implement universal use of face coverings.
Advise staff who are sick to stay home.
Encourage all prescribers to submit prescription orders via telephone or electronically.
Limit direct contact with customers.
Reduce risk during COVID-19 testing.
Maintain social distancing
Ensure that the waiting area is cleaned regularly.
Close self-serve blood pressure units.
- Development goals to support transformation of the entire pharmacy profession launched by FIP (fip.org)Pharmaceutical Workforce Development Goals (fip.org)
Goals to support the transformation of the pharmacy profession around the world are launched today by FIP...The FIP Development Goals build on 13 Pharmaceutical Workforce Development Goals for pharmacy education developed by the federation in 2016. Additions have been made to these 13 goals and eight new goals have been developed, providing a total of 21 goals relevant to fields of practice and science as well as to workforce and education...READ MORE
The new goals cover:
• Medicines expertise
• People-centred care
• Communicable diseases
• Antimicrobial stewardship
• Access to medicines, devices & services
• Patient safety
• Digital health
• Sustainability in pharmacy - Pharmacists Feel Overworked, Face More Discrimination (drugtopics.com)2019 National Pharmacist Workforce Study (aacp.org)
Pharmacists, pharmacy technicians, and other pharmacy staff felt overtaxed even before the coronavirus disease 2019 pandemic hit, according to findings from the 2019 National Pharmacist Workforce Study. The survey results showed that 69% of full-time pharmacists reported that their workload “increased” or “greatly increased” compared with the prior year...Although the profession is much more racially and gender diverse than in previous years, pharmacy staff reported age, race, and gender discrimination...Among full-time, actively practicing pharmacists, 71% rated their workload level at their primary place of employment as “high” or “excessively high” in 2019, compared with 66% and 68% of full-time pharmacists in 2014 and 2009, respectively...READ MORE
- Eyeing COVID-19 shortages, FDA unleashes compounded drugs to treat hospital patients (fiercepharma.com)
The FDA is easing its lockdown on compounded drugs to help ease COVID-19 drug shortages...After a series of high-profile failures in the early 2010s, the compounding pharmacy industry took its share of body blows from an FDA looking to impose its will...The FDA will temporarily allow hospitals to source hard-to-find drugs from compounding pharmacies to treat certain patients hospitalized with severe COVID-19...The regulation, meant to last as long as hospitals continue to encounter shortages of key drugs, applies to compounding pharmacies that aren't already sanctioned by the FDA as "outsourcing facilities." To qualify, the copycat drugs must be listed on the FDA's shortages list, and hospitals must have exhausted all other options to access a commercial version of the drug...READ MORE