- FDA issues draft guidance on compounding at outsourcing facilities (biopharmadive.com)
The FDA released a draft version of guidance covering compounding, called "Evaluation of Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry," directed primarily at outsourcing facilities, addressing the use and qualification of bulk substances in compounding...The proposed rules are an extension of The Drug Quality and Security Act...which identified outsourcing facilities as its own category, separate from traditional compounders...Active pharmaceutical ingredients must be accompanied by a monograph from an appropriate governing party (if a monograph exists), must be made in a facility that has prior approval, and must come with a certificate of analysis (to prove they been characterized)...The agency proposes two specific ways to tell if a compounded drug at an outsourcing facility is safe: whether attributes of the approved drug may make it unsuitable to treat certain patients for particular conditions (including whether the compounded drug is intended to address that attribute), and second, if certain factors for each substance being proposed for use in a compounded drug product – specifically, "its physical and chemical characterization, possible or known safety issues, evidence or lack of thereof of effectiveness, and historical use" — would preclude its use by a third-party facility...The agency says the plan will "clarify and appropriately tailor the policies for traditional compounding pharmacies and the outsourcing facilities that may supply a broader market."...
- FDA Releases 2018 Compounding Policy Priorities Plan (iacprx.org)
The Food & Drug Administration has released a 2018 Compounding Policy Priorities Plan...FDA also issued a final guidance on mixing, diluting, or repackaging biological products, which describes the conditions under which the agency does not intend to take action when certain biological products are mixed, diluted, or repackaged in a manner not described in their approved labeling. According to FDA, "These policies are intended to minimize public health risks, while preserving access to these products for patients who have a medical need for them."
- Exclusive: FDA plans new compounding pharmacy policy, agency head says (reuters.com)
The head of the U.S. Food and Drug Administration said...the agency is working on a new policy that would encourage more compounding pharmacies to register under...the Drug Quality and Security Act, which aimed to bring more compounding pharmacies...under the authority of the FDA rather than state pharmacy boards...The law created a category of “outsourcing facilities” that could register with the FDA, allowing them to sell products in bulk to hospitals and physician practices without prescriptions for individual patients...In exchange, those compounders would have to follow federal manufacturing standards and subject themselves to routine inspections...around 70 firms have registered as outsourcing facilities...compounders that did not register with the FDA would remain under state oversight, and...could only compound drugs based on prescriptions for specific patients...Gottlieb said that in order to encourage more compounders to register, the FDA would release draft guidance in the next two months reflecting its intention to adjust its enforcement priorities based on the size of registered compounders and the riskiness of their products...We’re looking at ways we can provide more of a gradation in our regulatory architecture so we don’t have a one-size-fits-all approach...
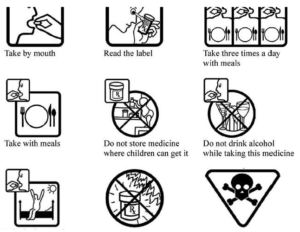
- New research reveals pictograms help seniors understand medication instructions (medicalxpress.com)
Nine different pharmaceutical pictograms that could help older people understand written medical information...Simple images designed to convey information about prescription drugs could help save lives and reduce the economic burden of non-adherence to treatment. New research...shows that including pictograms on written medication instructions helps seniors take their drugs correctly...Patients with multiple prescriptions can easily get confused and take the wrong medication, leading to hospitalization and even death…pictograms on a prescription drug label does help older people understand medical information and instructions. The pictograms provided information such as "take with meals" or "do not leave in direct sunlight" and warnings such as "poison" and "do not leave near children."...This not only prevents accidental overdose, it relieves some of the pressure that our aging population is putting on the health service by avoiding preventable tragedies...
- Drug compounding oversight rises, but spotty: Pew (biopharmadive.com)
State governments have stepped up their oversight of drug compounding in recent years, yet resource limitations may be hamstringing regulators from even more thorough inspections of traditional pharmacies, according to a new report...In 2015, the District of Columbia and 26 states required routine inspections of compounding pharmacies at least once a year. But by 2017, the number had fallen to 22 states plus the nation's capital...Many states have taken significant steps to better regulate drug compounding since the disastrous meningitis outbreak that came as a result of the New England Compounding Center's adulterated steroids...32 state boards of pharmacy require traditional pharmacies that compound sterile drugs to be in complete alignment with quality standards set forth in the U.S. Pharmaceutical Convention General Chapter. Another 11 states have provisions in place that they've determined are as strong — if not stronger — as the General Chapter's guidance...Issues have arisen despite that progress...Pew's research identified more than 50 reported or potential compounding errors from 2001 to 2017. The errors were linked to 1,227 adverse events, including 99 deaths — and those figures are likely low estimates, according to the organization.
- Pharmacist’s ‘deadly’ choices sparked U.S. meningitis outbreak: prosecutors (reuters.com)
A federal prosecutor told jurors...that a Massachusetts pharmacist gambled with patients’ lives by making drugs in unsafe ways that led to a deadly 2012 fungal meningitis outbreak, but a defense lawyer said he was no murderer...Glenn Chin, a former supervisory pharmacist at New England Compounding Center, made drugs in filthy conditions, producing mold-tainted steroids in the process...Those steroids were shipped out to healthcare facilities nationally and then injected into patients, leading to an outbreak that sickened 778 people, including 76 people who died…“Make no mistake, Glenn Chin is not sitting in this court room because he was negligent or careless,”... “He is here because of his deliberate choices.”...Chin directed “massive corner cutting” in...NECC’s so-called clean rooms where the drugs were made, prioritizing production over cleaning and failing to properly test or sterilize drugs.
- Pharmacy exec seeks new trial over role in deadly U.S. meningitis outbreak (reuters.com)
Lawyers for a Massachusetts pharmacy executive convicted of fraud for his role in a 2012 U.S. meningitis outbreak that killed 64 people asked a judge to order a new trial, charging that prosecutors misbehaved in providing evidence to the jury...Barry Cadden, co-founder of the now-defunct New England Compounding Center, was cleared of second-degree murder charges but was found guilty in March of racketeering and fraud for his role in shipping injectible steroids tainted with fungus linked to the deadly outbreak that also sickened 753 people in 20 states...Cadden's attorneys argued that prosecutors overreached in the number and severity of criminal charges that they filed against him. The attorneys said the prosecutors misled the jury by providing them a binder filled with laboratory tests showing that vials of steroids shipped by NECC were tainted but not providing comparable reports submitted by defense attorneys showing the vials were sterile.
- Pharmacist tied to U.S. meningitis outbreak gets eight years in prison (reuters.com)
A Massachusetts pharmacist was sentenced...to eight years in prison after being convicted on racketeering and fraud charges stemming from his role in a 2012 fungal meningitis outbreak that killed 76 people and sickened hundreds more...Glenn Chin, the former supervisory pharmacist at New England Compounding Center, was convicted by a federal jury in Boston in October but was cleared of second-degree murder charges, which would have exposed him to a maximum prison sentence of life...Prosecutors had asked U.S. District Judge Richard Stearns to sentence Chin...to 35 years in prison for overseeing the dispensing of substandard drugs made in filthy conditions at the now-defunct...NECC...Prosecutors said those drugs included mold-tainted steroids...that were then injected into patients, harming at least 793 people in 20 different states...
- Pharmacists Helping Others – Hurricane Irma Resources (myemail.constantcontact.com)
As many find themselves dealing with the devastating aftermath of yet another hurricane, the International Academy of Compounding Pharmacists wanted to highlight members who are working around the clock to help patients and other pharmacists, as well as provide you with invaluable pharmacy and legal resources that are available for you..
- GAO Releases Compounding Report (iacp.site-ym.com)
The U.S. Government Accountability Office has published a report...on compounding entitled “ Drug Compounding – FDA Has Taken Steps to Implement Compounding Law, but Some States and Stakeholders Reported Challenges.”