- CVS Health asks Gov. Scott to veto contentious pharmacy bill (vtdigger.org)H.353 (legislature.vermont.gov)
CVS Health has asked Gov. Phil Scott to veto H.353 — a last-ditch effort to block a bill it says would raise prescription drug costs for Vermonters with private insurance...H.353 started out as a bill to make drugs more affordable by regulating pharmacy benefits managers, third-party companies that negotiate medication coverage plans for consumers with private insurers. But revisions of the bill, as it worked its way through the House and Senate, would have all but guaranteed that specialty drug prescriptions given to patients in health care settings, including expensive cancer medication, would be filled at the University of Vermont Health Network’s pharmacy in Burlington, rather than through cheaper mail-order pharmacies that insurers prefer...READ MORE
- Expanding Access Takes Telepharmacy to the Next Level (drugtopics.com)
During the COVID-19 pandemic many hospitals and health care services teams turned to telepharmacy to reduce delays in providing medications to patients while social distancing practices were in place. Hospitals quickly found that telepharmacy benefitted pharmacy services by providing patients with more efficient access to medical care and reducing costs. Thus, hospitals were able to focus more on patient care and less on logistics, which led to higher turnaround times and increased patient satisfaction...READ MORE
- Medication Errors: 2019, The Year in Review – January to December 2019 (pharmacypracticenews.com)
- Safety Issues Related to Labeling, Packaging, and Nomenclature
- Safety Issues Associated With Order Communication and Documentation
- Problems Involving Drug Information, Patient Information, Patient Education, and Staff Education
- Safety Issues Related to Medical Devices and Equipment
- Other Discussion Items
- SMP’s Targeted Medication Safety Best Practices for Hospitals...READ MORE
- Health Systems Keep Pushing into Specialty, But Roadblocks Remain (pharmacypracticenews.com)
Hospitals and health systems are building and expanding their own specialty pharmacies, and with good reason, according to several veterans of specialty rollouts...more than 75% of hospitals with at least 600 beds operate a specialty pharmacy, allowing them to directly prescribe patients critical medications for various chronic and often life-threatening diseases...An in-house specialty pharmacy provides many financial and clinical advantages over external specialty pharmacies for both patients and providers...significant roadblocks, and time and effort needed, to successfully launch a specialty pharmacy...READ MORE
- New frontiers in pharmacy education: Preparing students for their role in an evolving pharmacy practice (drugstorenews.com)
...the new crop of pharmacy students nationwide will need much more than an introduction to rudimentary science courses, thanks to the notable progress that the pharmacy industry has made in advancing the clinical role of pharmacists. The pandemic also has elevated pharmacists’ roles and allowed them to assume expanded responsibilities...Many pharmacy schools are stepping up to the plate with new courses and electives, as well as honing some of their existing courses, to ensure that their students are well prepared to meet the myriad challenges that they will face as newly minted pharmacists...READ MORE
- White House says data is one key to improving pharma supply chain resilience (healthcareitnews.com)
Other recommendations include boosting local production, promoting research and development, and creating robust quality management maturity...The White House and several federal agencies...released a series of policy recommendations for addressing the vulnerabilities in U.S. pharmaceutical supply chains...The review came in response to an executive order signed by President Joe Biden...that directed the government to identify risks, address vulnerabilities and develop a strategy to promote supply chain resilience throughout sectors...READ MORE
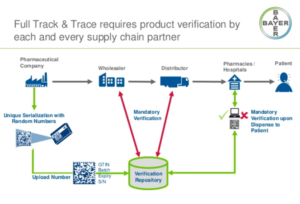
- Track-and-trace requirements go into effect on Friday (pharmacist.com)
Starting Friday, November 27, 2020, pharmacies must buy and sell only products with a required “product identifier” on their packages. This requirement is part of the phased-in implementation of the Drug Supply Chain Security Act of 2013, also known as the “track-and-trace” law. By Friday, dispensers should be familiar with this requirement and know what to do if a product identifier is not on the package when they receive products that require it. The product identifier is on most drug packages in both human-readable format and on a machine-readable 2D data matrix barcode...“The challenge for dispensers is that not all drug product packages are required to have a product identifier, and there is no central database to check if a product should have one,”...READ MORE
- Few Rx supply chain stakeholders prepared to share DSCSA-required transaction data (chaindrugreview.com)
Results from a new HDA Research Foundation survey indicate that the pharmaceutical supply chain is entering a critical phase in achieving the transaction data connections required to comply with the Drug Supply Chain Security Act in 2023...The DSCSA requires transaction data with product identifiers to be provided with physical product on November 27, 2023...“many healthcare supply chain trading partners are realizing there is work to be done to establish proper business-to-business connections; ensure data are formatted, transmitted and received successfully; that processes for troubleshooting are created; and that products in inventory have the right data attached to them for shipping after November 27,” said Perry Fri...COO of the HDA Research Foundation. “The Foundation’s survey shows...what might be leading to slow implementation rates across the supply chain.”...READ MORE
The uncertainty of coronavirus disease 2019 caused anticipatory purchasing of medications around the world, driving demand to an unprecedented high. Meanwhile, drug factories shut down in order to prevent the spread of COVID-19, the drug-supply chain was disrupted, and drug shortages resulted. In the face of these drug shortages, pharmacy personnel responded by initiating local policy changes, implementing detailed antibiotic stewardship, and enacting quantity limits for in-demand medications...READ MORE
- Pharmacies must comply with certain track-and-trace requirements by November 27, FDA says (pharmacist.com)
Despite APhA’s concerns that compliance with certain Drug Supply Chain Security Act requirements undermines pandemic response efforts, FDA has confirmed that “dispensers,” including pharmacies, must meet a requirement that goes into effect on November 27, 2020. APhA and pharmacy partners had requested that FDA delay enforcement of certain portions of the DSCSA—also known as “track and trace”—mandate...READ MORE