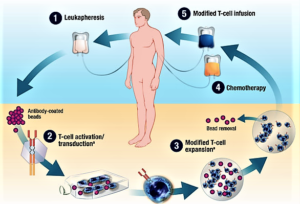
- The First CAR-T Drugs Have Left the Gate (fool.com)
Investors should keep an eye on this promising way to treat cancer...For all the talk about biotechs being nimble, it's a big pharma that looks like it'll be the first company to launch a chimeric antigen receptor T-cell (CAR-T) product...Novartis announced last week that the Food and Drug Administration accepted its application to market tisagenlecleucel-T...in patients with B-cell acute lymphoblastic leukemia who are relapsed and refractory to other therapies...A few days later, Kite Pharma completed its application for axicabtagene ciloleucel...Kite's application could be accepted early, putting it less than two months behind Novartis…Since CAR-T therapies are personalized treatments that have to be made individually for each patient, they're likely to be expensive to produce and therefore require a premium price. The first company to get a CAR-T therapy approved will set the price, which later companies may have to match unless they can justify a higher price with higher efficacy...With prices that will probably exceed those of current cancer treatments, investors should expect some pushback from insurers. One way Novarits and Kite can get around the cost issue is by offering money-back guarantees...Kite's and Novartis' CAR-T therapies are just the tip of the iceberg for this new way to treat cancer...
- Sanofi science chief on Zika: It’s time to disrupt traditional vaccine development (fiercevaccines.com)
The World Health Organization has warned that the Zika R&D frenzy may not culminate in a vaccine in time for the current outbreak, but Sanofi Chief Scientific Officer Gary Nabel won't take that for an answer. Nabel says it can be done, but it means turning the traditional vaccine development model on its head...In an interview...Nabel highlighted that the classic response to emerging and infectious diseases, such as MERS and Ebola, has been inadequate...We just run from one crisis to another…That's no way to protect the world's population...The traditional vaccine development timeline typically takes 5 years or more to produce a marketable vaccine... To have a chance at accelerating this for emerging diseases, biotechs and pharmas, regulators and government agencies need to come together and unpack this model, identifying where time can be saved and exploring different ways to go about clinical trials...(Nabel) identified WHO as "symptomatic of the problem." While it has "the best of intentions," it doesn't have the wherewithal to follow through...The agency has declared Zika a global public health emergency and in February called for $56 million in funding to combat the virus. Just over a month later, only $3 million--5%--in funding has trickled in...