- A serious new hurdle for CRISPR: Edited cells might cause cancer, two studies find (statnews.com)CRISPR stocks tank after research shows edited cells might cause cancer (cnbc.com)
Editing cells’ genomes with CRISPR-Cas9 might increase the risk that the altered cells, intended to treat disease, will trigger cancer, two studies published on Monday warn — a potential game-changer for the companies developing CRISPR-based therapies...scientists found that cells whose genomes are successfully edited by CRISPR-Cas9 have the potential to seed tumors inside a patient. That could make some CRISPR’d cells ticking time bombs...The CEO of CRISPR Therapeutics, Sam Kulkarni, told STAT the results are “plausible.” Although they likely apply to only one of the ways that CRISPR edits genomes (replacing disease-causing DNA with healthy versions) and not the other (just excising DNA), he said, “it’s something we need to pay attention to...We need to do the work and make sure edited cells returned to patients don’t become cancerous.”...Standard CRISPR-Cas9 works by cutting both strands of the DNA double helix. That injury causes a cell to activate a biochemical first-aid kit orchestrated by a gene called p53, which either mends the DNA break or makes the cell self-destruct...The flip side of p53 repairing CRISPR edits, or killing cells that accept the edits, is that cells that survive with the edits do so precisely because they have a dysfunctional p53 and therefore lack this fix-it-or-kill-it mechanism...The reason why that could be a problem is that p53 dysfunction can cause cancer...P53 mutations are responsible for nearly half of ovarian cancers; 43 percent of colorectal cancers; 38 percent of lung cancers; nearly one-third of pancreatic, stomach, and liver cancers; and one-quarter of breast cancers...
- Lab-Grown Mini Organs Could Speed Up Drug Discovery (forbes.com)
The thought of lab-grown organs conjures up Frankenstein-like imagery. The reality however, is somewhat less visually dramatic, with the term ‘organoids’ used to describe tiny 3D structures of human tissue, a millimeter or so in diameter...these tiny lumps of cells are creating a lot of excitement in the world of medical research...Cells in dishes and animal models have been used for preclinical testing of drugs for decades. Success in these experiments is a key hurdle for any new medicine to overcome before being given the green light for all-important human clinical trials...Organoids are most commonly made either from a small sample of tissue needled out of a person or from stem cells cultured in a cocktail of nutrients intent on pushing them towards becoming a particular tissue type. So far, organoids have been made resembling several tissues including lung, liver, brain, kidney and intestine...as a relatively new innovation they are being used to investigate dozens of conditions from infectious diseases to cancer.... A study published last year in Science Translational Medicine by scientists at the University Medical Centre, Utrecht generated organoids formed from the rectal tissue of 71 people with cystic fibrosis and exposed them to experimental drugs. By observing changes in the organoids, the scientists accurately predicted which patients would respond to the therapies in just one week at a cost of around $1200 per patient. The results were so convincing that a positive organoid test is now considered sufficient evidence for insurance companies to fund the new therapies in the Netherlands...
- IBM pitched its Watson supercomputer as a revolution in cancer care. It’s nowhere close (statnews.com)
It was an audacious undertaking, even for one of the most storied American companies: With a single machine, IBM would tackle humanity’s most vexing diseases and revolutionize medicine...promoting its signature brand — Watson — IBM sought to capture the world’s imagination, and it quickly zeroed in on a high-profile target: cancer...But three years after IBM began selling Watson to recommend the best cancer treatments to doctors around the world, a STAT investigation has found that the supercomputer isn’t living up to the lofty expectations IBM created for it. It is still struggling with the basic step of learning about different forms of cancer. Only a few dozen hospitals have adopted the system, which is a long way from IBM’s goal of establishing dominance in a multibillion-dollar market...While it has emphatically marketed Watson for cancer care, IBM hasn’t published any scientific papers demonstrating how the technology affects physicians and patients. As a result, its flaws are getting exposed on the front lines of care by doctors and researchers who say that the system, while promising in some respects, remains undeveloped...
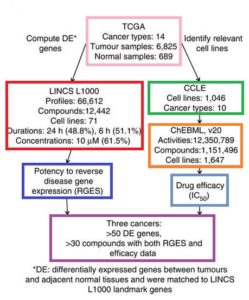
- Big-Data Analysis Points Toward New Drug Discovery Method (technologynetworks.com)Reversal of cancer gene expression correlates with drug efficacy and reveals therapeutic targets (nature.com)
A research team led by scientists at UC San Francisco has developed a computational method to systematically probe massive amounts of open-access data to discover new ways to use drugs, including some that have already been approved for other uses...The method enables scientists to bypass the usual experiments in biological specimens and to instead do computational analyses, using open-access data to match FDA-approved drugs and other existing compounds to the molecular fingerprints of diseases like cancer. The specificity of the links between these drugs and the diseases they are predicted to be able to treat holds the potential to target drugs in ways that minimize side effects, overcome resistance and reveal more clearly how both the drugs and the diseases are working...Our hope is that ultimately our computational approach can be broadly applied, not only to cancer, but also to other diseases where molecular data exist, and that it will speed up drug discovery in diseases with high unmet needs...I’m (Bin Chen, PhD) most excited about the possibilities for applying this approach to individual patients to prescribe the best drug for each...
- FDA commissioner to health insurers: You’re doing it wrong (cnbc.com)
Insurance is designed, theoretically, to protect against the catastrophic: tornadoes, floods, hurricanes — or, where our health is concerned, cancer or another devastating disease...To make that financial protection affordable, many pay into the system: the healthy are supposed to subsidize the sick...But at a conference...organized by the health insurance industry, FDA Commissioner Scott Gottlieb delivered a startling message: You're doing it wrong..."Sick people aren't supposed to be subsidizing the healthy," Gottlieb told an audience at the National Health Policy Conference of AHIP, the health insurer industry group. "That's exactly the opposite of what most people thought they were buying when they bought into the notion of having insurance."...Gottlieb's remarks were focused on the health of the market for biosimilars — copycats of complex, biologic medicines — and his concerns that industry consolidation and what he called rigged payment schemes may be stifling their development.
- China may relax trial requirements for new drugs, allowing foreign data (fiercebiotech.com)
China has said it will accept data from clinical trials run overseas in a bid to shorten the time it takes to approve new drugs and medical devices...It’s the latest in a string of regulatory initiatives implemented by Chinese authorities that could be a boost for international biopharma companies as well as Chinese patients, who sometimes have to wait six or seven years after launch in Western markets for drugs to be launched in China...The country’s huge and increasingly affluent population is facing an ever-growing burden of chronic diseases like cancer and diabetes, and the government has been under pressure to improve access to healthcare and new medicines...The latest wide-ranging set of proposals...recognizes that China is lagging behind other countries when it comes to approving new drugs. It’s approved 100 innovative new drugs in the last five years, around a third the number in developed markets...
- Could indication-based pricing really work? (biopharmadive.com)
Why should it cost more than 30 times as much for essentially the same drug just because it’s being used to treat an eye condition rather than cancer?...So-called indication-specific pricing may seem absurd to some observers but makes perfect sense to industry insiders. Drugs are often approved to treat several different diseases, often with varying patient population sizes. For example, Allergan plc's Botox (onabotulinumtoxin A) is approved to treat more than eight indications, including wrinkle reducing and migraines. While insurers may choose to cover these indications differently, indication-based could mean these two indications are priced at opposite ends of the pricing spectrum, for example, and could better align reimbursement with value...There are plenty of examples of drugs approved for wildly different patient populations. Yet, so far, indication-based pricing is not a reality in the U.S.
- UN: About 11 percent of drugs in poor countries are fake (ktvn.com)
About 11 percent of medicines in developing countries are counterfeit and likely responsible for the deaths of tens of thousands of children from diseases like malaria and pneumonia every year, the World Health Organization said...It's the first attempt by the U.N. health agency to assess the problem. Experts reviewed 100 studies involving more than 48,000 medicines. Drugs for treating malaria and bacterial infections accounted for nearly 65 percent of fake medicines... Between 72,000 and 169,000 children may be dying from pneumonia every year after receiving bad drugs. Counterfeit medications might be responsible for an additional 116,000 deaths from malaria mostly in sub-Saharan Africa...Counterfeit drugs include products that have not been approved by regulators, fail to meet quality standards or deliberately misrepresent an ingredient...In 2013, WHO set up a voluntary global monitoring system for substandard and fake drugs and has received reports of about 1,500 problematic medicines including drugs that claim to treat heart problems, diabetes, fertility problems, mental health issues and cancer. WHO also reported problems of fake vaccines for diseases including yellow fever and meningitis...
- Express Scripts: Specialty meds driving up US drug spend (biopharmadive.com)
An estimated three out of every 1,000 Americans ring up a bill of more than $50,000 in annual prescription drug costs last year, according to a new analysis of health plan members published...by Express Scripts... The number of people who meet this threshold for high pharmaceutical expenditures has risen sharply, by 35%, since the PBM's last report on the trend in 2014. While numerically small, this group of patients accounted for more than 20% of total U.S. spending on prescription drugs in 2016...higher utilization of pricey specialty medicines for cancer, multiple sclerosis and rare diseases was the primary driver of spending among patients with annual pharmacy costs over $50,000. Yet that was a change from 2014, when new hepatitis C drugs and compounded medicines goosed spending...Nearly 96% of the plan members who cleared the $50,000 in annual spending threshold used specialty medicines, most notably cancer therapeutics. While the average annual cost of oncology drugs was lower than some other specialty categories, a quarter of all spending by this group was on cancer treatments...
- Trump’s FDA Commissioner on Drug Prices, Regulations, Science (bloomberg.com)
Trump vs. Big Pharma: Can He Bring Drug Prices Down?...U.S. Food and Drug Administration Commissioner Scott Gottlieb spoke with Bloomberg News about drug pricing, new medicine and regulations. This transcript of the interview has been edited for clarity and length.
- What’s the FDA’s role to play in drug pricing and what can the agency do, given that it hasn’t traditionally had a mandate to address the issue?
- Is it just small, opportunistic drug companies that are "gaming" the system, or is this something bigger companies do as well?
- What about the rest of the administration? Trump talked a lot about drug pricing on the campaign trail and after, yet we haven’t seen much action other than yours.
- What about an executive order on drug pricing -- there was talk that Trump was going to come out with something. Is that still being worked on?
- What about drugs like EpiPen, would you come out with new rules there to create more competition? [EpiPen, made by Mylan NV, is what’s known as a drug-device combination, where both the medicine and the device that administers it can have patent protections.
- But is it reasonable to assume you’re looking at doing something like this, on these types of devices?
- One of the things we’ve seen from the administration is, get rid of regulation, get rid of regulation, get rid of regulation. How does your philosophy as a regulator –- one of the biggest regulators in the U.S. government –- how does that line up with what the Trump administration has called for?
- Some of the things you’re doing to create more competition among drugs, people could interpret as a loosening of standards. I wonder if you think that’s the case?
- Through the years we’ve seen the agency go through cycles, of pushing drugs out into the market faster, versus being much more conservative about safety. Is the balance at the FDA changing?
- How broadly can you apply new standards or guidelines for drug approval? There’s been talk about it in cancer, but what about other diseases?