- Gottlieb Proposes Modernization of Drug Review Office (biopharminternational.com)
...FDA Commissioner Scott Gottlieb, MD, announced that the Center for Drug Evaluation and Research would be taking steps to modernize the organization and functions of the Office of New Drugs in order to address scientific and medical advances within the industry. The goal is to make the review process more integrated across science and regulatory expertise. Janet Woodcock, director of CDER, plans on elevating the role of FDA scientists and medical officers and providing these officers with more tools and support “to advance the clinical and regulatory principles that the FDA uses to evaluate new drugs for safety and efficacy.”...Other changes will include the development of guidance documents, giving review staff more time with sponsors, and getting sponsors involved earlier in the development process. Engaging disease specialists, academic researchers, regulatory partners at other agencies, and patient groups is also a goal of CDER.
- FDA Details Plans for More Efficient Inspections, Facility Evaluations
The US Food and Drug Administration's Center for Drug Evaluation and Research and Office of Regulatory Affairs will soon launch an effort to streamline the two offices' inspection and facility evaluation efforts...CDER Director Janet Woodcock and Associate Commissioner for Regulatory Affairs Melinda Plaisier said it is vital that the two offices quickly implement the plan in order to meet commitments under the recently reauthorized user fee agreements, specifically citing the agency's promise to communicate final inspection classifications to generic drugmakers within 90 days of an inspection beginning in October 2018...We plan to operationalize the plan in the fall of 2017 for nearly all human drugs...FDA details the plan—which includes specific operating models for pre- and post-approval inspections, surveillance inspections and for-cause inspections—in a 20-page white paper obtained by Focus entitled Integration of FDA Facility Evaluation and Inspection Program for Human Drugs: A Concept of Operations...
- CDER Publishes Drug Safety Report – FDA’s Center for Drug Evaluation and Research has published its second annual report on key safety programs and activities. (biopharminternational.com)
...FDA’s Center for Drug Evaluation and Research released its second annual Drug Safety Priorities report, which details drug safety initiatives carried out by CDER and FDA. The report highlights drug safety program milestones and gives an update on goals achieved in 2017. Efforts by FDA to ensure drug safety science, surveillance, and oversight are discussed...Detailed in the report are the agency’s efforts on pharmacovigilance, medication errors, and risk management...The report also goes into detail about the agency’s views on how real-world evidence can advance drug safety. An update on the agency’s efforts to combat the opioid crises is also provided...
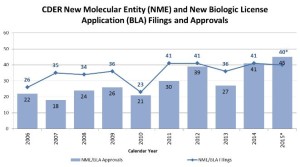
- 2015: Another Strong Year for Patients in Need of New Drug Therapies (blogs.fda.gov)Novel New Drugs Summary 2015 (fda.gov)I’m (John Jenkins,Director of the Office of New Drugs) pleased to report another strong year for FDA approvals of novel new drugs, which offer many patients new treatment options for serious and life-threatening conditions. In 2015, FDA’s Center for Drug Evaluation and Research approved 45 novel new therapies – significantly more than the average of 28 we have approved during the previous nine years of this decade...During this past year, we approved many new drugs to treat various forms of cancer, including four to treat multiple myeloma, and others to treat lung, skin, breast, brain, colorectal, and other cancers. We also approved new drugs to treat heart failure, high cholesterol, cystic fibrosis, and irritable bowel syndrome, as well as the first approved reversal agent for a commonly-used blood thinner...Here are a few highlights of these approvals:
- More than one-third of the novel new drugs CDER approved in 2015 were identified by FDA as “first-in-class,” for example, drugs that use a new and unique mechanism of action for treating a medical condition;
- More than 40% of these new therapies were approved to treat rare or “orphan” diseases that affect 200,000 or fewer Americans–Americans who often have few or no drug treatment options;
- 60% of CDER’s novel new approvals for 2015 were designated in one or more categories of Fast Track, Breakthrough, Priority Review, or Accelerated Approval. Each of these designations helps speed the development and/or approval process and is designed to help bring important medications to the market as quickly as possible; and
- 64% of CDER’s novel new approvals were approved first in the United States before any other country.
- Will Pharma Meet the Drug Tracking Deadline? (biopharminternational.com)
Manufacturers and trading partners struggle to meet drug tracking requirements...As FDA and industry near the halfway mark in the 10-year process for establishing a national electronic drug tracking system by 2023, there’s considerable concern among pharma companies, wholesaler/distributors, and pharmacists about meeting the deadline. The process for establishing the rules and infrastructure for the track-and-trace system envisioned in the Drug Supply Chain Security Act, part of the Drug Quality and Security Act of 2013, is proving to be complex and challenging for all parties...FDA recently delayed requiring drug manufacturers to imprint unique product identifiers on individual packages by November of 2017, saying it would not enforce that policy until Nov. 27, 2018. While major pharma companies are meeting the earlier time-frame for serializing and identifying drug packages, many generic-drug makers and contract manufacturers reported confusion over who is responsible for devising the identifiers and for confirming compliance with requirements. And while manufacturers applauded gaining an additional year to fully identify individual drug packages, pharmacists and other supply chain partners raised concerns that the delay would make it even more difficult for them to comply with reporting requirements for 2018 and 2019...
- Why Partnerships are Key to the Science of Patient Input (blogs.fda.gov)
We recently announced…Patient Engagement Advisory Committee …The Committee will provide advice to the FDA Commissioner on…issues relating to medical devices, the regulation of devices, and their use by patients…PEAC will bring patients, patient advocacy groups, and experts together for a broader discussion of important patient-related issues, to increase integration of patient perspectives into the regulatory process, and to help drive more patient-centric medical device innovation, development, evaluation, and access.