- CMS reportedly proposes 2-year ban of Theranos founder Holmes, revocation of lab’s license (biopharmadive.com)Walgreens Is Reportedly Taking Steps to Dump Theranos (fortune.com)
Centers for Medicare and Medicare Services is reportedly proposing to ban Theranos founder Elizabeth Holmes from owning or operating a laboratory for two years, along with revoking the license of Theranos' Newark, CA lab...Theranos had ten calendar days from receiving the notice to explain to CMS why the sanctions should not be imposed on Holmes and the company...The proposed sanctions stem from a number of key deficiencies CMS found in inspections last fall. Theranos had sent in a correction plan for the deficiencies but the regulator found it to be not credible.
- Medicaid Pharmacy Reimbursement Changes Coming Soon (ashp.org)
Centers for Medicare and Medicaid Services late this month plans to publish a long-awaited official revision to the maximum allowable reimbursement amount for multiple-source outpatient drugs covered by state Medicaid programs...The revised amounts, known as federal upper limits, will become effective April 1 to coincide with provisions in the Covered Outpatient Drugs final rule...FULs were most recently updated in September of 2009...Starting in April, the agency plans to update the FULs monthly. State Medicaid plans will have up to 30 days to implement each set of new FULs...state Medicaid plans have flexibility to implement the new reimbursement model, as long as the state's plan is based on pharmacies' true drug acquisition costs...states have flexibility in setting their professional dispensing fees, including whether to use state or national data to calculate rates...CMS expects state Medicaid plans to provide "credible data" that demonstrates how their reimbursement plan accurately accounts for dispensing costs...States must consider the totality of reimbursement to pharmacies when they are looking at changes in either the ingredient cost or professional dispensing fee...State Medicaid agencies have until June 30, 2017, to submit their amended plans to CMS. The revised plans must go into effect by April 1, 2017.
- Health exchange disputes federal report (reviewjournal.com)
Nevada's health insurance exchange disputes a federal report claiming it misallocated funds for creating its program and recommending it consider refunding $893,000 to the Centers Medicare and Medicaid Services...A refund is not needed, the Silver State Health Insurance Exchange stated in a written response to the report from the Office of Inspector General, Department of Health and Human Services...We believe that this conclusion is based upon an erroneous interpretation of federal guidance regarding cost allocation accounting principles and the timing of cost allocation adjustments...It also pointed to comments from CMS that not only back up the state agency's take on the matter but are also included in the OIG's own report...In its report, however, the OIG was not swayed, stating it believes "our first recommendation is valid." The OIG cited the Nevada agency for using outdated data when it was establishing its marketplace despite the availability of updated data, having no internal controls, allowing insufficient staff oversight, and having no written policy on the allocation process...The Nevada marketplace should have used the updated, better data to update its cost allocation methodology...
- CMS final rule addresses decades-long Medicaid reimbursement issue (drugstorenews.com)
The end of what has become a decades long battle around fair reimbursement for Medicaid programs and pharmacy appears to be in sight...the Centers for Medicare & Medicaid Services issued the Covered Outpatient Drugs final rule with comment that addresses key areas of Medicaid drug reimbursement and changes made to the Medicaid Drug Rebate Program by the Affordable Care Act. According to a fact sheet published by CMS, this final rule assists states and the federal government in managing drug costs, establishes the long term framework for implementation of the Medicaid drug rebate program and creates a more fair reimbursement system for Medicaid programs and pharmacies...the final rule is designed to ensure that pharmacy reimbursement is aligned with the acquisition cost of drugs and that the states pay an appropriate professional dispensing fee. The final rule:
- Creates an exception to the FUL calculation, which allows for the use of a higher multiplier than 175% to calculate the FUL based on acquisition costs for certain multiple source drugs;
- Establishes actual acquisition cost as the basis by which states should determine their ingredient cost reimbursement so payments are based on a more accurate estimate of the prices available in the marketplace, while still ensuring sufficient beneficiary access;
- Implements the use of the term professional dispensing fee to ensure that the dispensing fee paid to pharmacies reflect the cost of the pharmacist’s professional services and cost to dispense the drug product to a Medicaid beneficiary;
- Clarifies that states are required to evaluate the sufficiency of both the ingredient cost reimbursement and the professional dispensing fee reimbursement when proposing changes to either of these components; and
- Requires states to specify in the Medicaid state plan that reimbursement methodology to pharmacies that purchase drugs through the Federal Supply Schedule and the 340B Drug Pricing Program is consistent with overall AAC requirements.
- Medicare ‘hospital star rating’ may correspond to patient outcomes (reuters.com)
The Centers for Medicare and Medicaid Services has been letting patients grade their hospital experiences, and those "patient experience scores" may give some insight into a hospital’s health outcomes...Some people have been concerned that patient experience isn’t the most important factor to measure...Medicare has been putting a lot of data out for a long time, but the broad consensus has been it’s very hard for consumers to use this info...CMS responded by giving out star ratings that consumers can understand easily...The five-star rating system is based on patients’ answers to 27 questions about a recent hospital stay...If you use the star rating you’re more likely to end up at a high quality hospital...But I wouldn’t use only the star rating to choose a hospital...
- Surprise proposal: Medicare wants to jump on the value-based pricing bandwagon (fiercepharma.com)
Doctors and cancer clinics are up in arms about a new Medicare reimbursement scheme that would cut their mark-ups on oncology drugs. But the Centers for Medicare and Medicaid Services has even bigger plans for cancer-drug payments...Pay-for-performance deals and indication-specific reimbursements are on a list of 6 programs CMS plans to try alongside the cuts targeted at physicians and hospitals. It's a rare example of Medicare plucking new ideas from the private sector, even before they've been widely adopted in the biopharma industry...CMS says it looked to private payers for "value-based purchasing tools," and wants to use strategies similar to those used by commercial health plans, pharmacy benefits managers, hospitals and other benefits managers...the agency would experiment with the sort of value-based reimbursement plans that Novartis and Amgen are using on their brand-new heart drugs Entresto and Repatha. CMS says it will be seeking "risk-sharing" deals with drugmakers to link drug payments with patient outcomes...In practice, performance-based deals can be difficult to administer, and that's one reason why U.S. payers have been reluctant to make pay-for-performance arrangements on Entresto...A U.K. government report found that the National Health Service had fallen short on clawing back rebates owed under its cost-sharing deals with drugmakers.
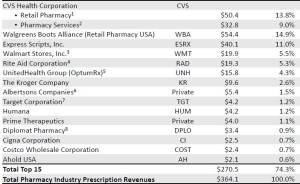
- The Top 15 Pharmacies of 2015 (drugchannels.net)Largest U.S. Pharmacies Ranked by Total Prescription Revenues, 2015 (pembrokeconsulting.com)
Next week, Drug Channels Institute will release our updated, revised, and expanded 2016 Economic Report on Retail, Mail, and Specialty Pharmacies...provides a sneak peek at the largest pharmacies, ranked by total U.S. prescription dispensing revenues for calendar year 2015…We estimate that total revenues of retail, mail, and specialty pharmacies reached $364.1 billion in 2015, up 12.1% from 2014. The top tier of dispensing pharmacies—CVS Health, Walgreens Boots Alliance, Express Scripts, Walmart, Rite Aid, and UnitedHealth Group’s OptumRx—accounted for about 64% of U.S. prescription dispensing revenues in 2015...many of the largest pharmacies are now central-fill, mail and specialty pharmacies operated by such PBMs and payers as Express Scripts, Caremark, and UnitedHealth. This reflects the growing role of specialty drugs in the pharmacy industry. We estimate that specialty drugs account for 35% or more of revenues at these pharmacies.
- ICD-10 to get 5,500 new codes, including ones for face, hand transplants, CMS says (healthcareitnews.com)
CMS said it plans to add about 1,900 diagnosis codes and 3,651 hospital inpatient procedure codes to the coding system…On Oct. 1, the Centers for Medicare and Medicaid Services will add another 5,500 codes to the ICD-10 diagnostic library, officials announced…The addition will come exactly one year after ICD-10, with its nearly 70,000 billable codes, replaced the dated, and much more compact, ICD-9 code set… The new and revised ICD-10-CM (Clinical Modification) and ICD-10 PCS (Procedure Coding System) codes will be included in the hospital inpatient prospective payment system proposed rule for fiscal 2017…
- CMS Seeks to Improve Access to Preferred Cost Sharing Pharmacies (specialtypharmacytimes.com)
Centers for Medicare Medicare & Medicaid Services is prioritizing an effort to make preferred cost sharing pharmacies available in geographical areas that have little access...we heard concerns that some beneficiaries did not have ready geographic access to preferred cost-sharing pharmacies...Increasingly, Part D plans are creating smaller networks of pharmacies within their larger networks and offering lower cost-sharing arrangements to beneficiaries who use these preferred cost-sharing pharmacies...Although plans are marketed with lower cost-sharing arrangements, there are some areas that do not have pharmacies accessible to beneficiaries...In order to address this issue, CMS laid out a plan to help in these areas...The plan includes measures such as:
- Working with outlier plans that addressed concerns regarding marketing and access
- Require the discloser in marketing material of plans offering less access to preferred cost-sharing pharmacies
- Publish access levels that offer a benefit structure for each plan.
- Deficiencies Found at Theranos Lab (wsj.com)
Federal inspectors will soon release details on problems at blood-testing facility...U.S. health inspectors have found serious deficiencies at Theranos Inc.’s laboratory in Northern California...The problems were found during an inspection by the Centers for Medicare and Medicaid Services, the chief federal regulator of clinical labs, at the blood-testing company’s facility in Newark, Calif. Failing to fix the problems could put the Theranos lab at risk of suspension from the Medicare program...inspection results are expected to be publicly released soon...