- Reimbursement Policy for Biosimilars Will Have Negative Consequences for Patients (americanactionforum.org)
Centers for Medicare and Medicaid Services have finalized a rule regarding the Medicare reimbursement methodology for biosimilar products. Biosimilars…approved …as being “highly similar” to a specific biologic medication…patients may respond differently to the reference product and the biosimilars...there is now a debate as to whether or not biosimilars should be paid with a single billing code based on the ASP (Average Sales Price) of all biosimilars for a single reference product, as has been finalized by CMS, or the ASP for each individual biosimilar, separately from any other biosimilar of the same reference product…Economic arguments and patient safety concerns may support the latter, though the statutory text regarding this matter is somewhat ambiguous…the decision over how biosimilars…should be reimbursed…should be determined by economic principles based on the value of the medication to patients and without putting patient safety and access to such products at risk. If the statutory text does not clearly provide for the favorable regulatory outcome of these factors, it should be amended.
- CMS warns state Medicaid programs on hepatitis c drug restrictions (pharmalot.com)
…the Obama administration wrote state Medicaid programs that they may be violating federal law by restricting access to hepatitis C medicines. At the same time, the Centers for Medicare and Medicaid Services wrote four drug makers asking for information about pricing arrangements with insurers and pharmacy benefits managers… A new crop of hepatitis C treatments have factored heavily in the conversation, thanks to very high cure rates but also high price tags… Ever since the new hepatitis C medications arrived nearly two years ago, public and private payers have called them budget-busters...some state Medicaid programs began restricting access based on a number of factors…CMS officials warned the state Medicaid programs against “imposing conditions for coverage that may unreasonably restrict access” to hepatitis C drugs. Placing restrictions may be “contrary to the statutory requirements” of a federal law that requires state Medicaid programs to pay for all medically necessary treatments…
- Rite Aid, Rx orgs make case for expanded MTM (drugstorenews.com)
...House of Representatives’ Committee on Energy and Commerce Subcommittee on Health convened a hearing called "Examining Medicare Part D Medication Therapy Management Program."...aimed at figuring out the best ways to strengthen MTM model as the Centers for Medicare and Medicaid roll out an overhaul… Rite Aid’s director of field clinical services… spoke in favor of expanding MTM…and highlighted recent efforts...which include the introduction of a bill…the Medication Therapy Management Empowerment Act that would increase access to MTM for patients with such chronic conditions as diabetes, cardiovascular disease, high cholesterol and COPD…given community pharmacist’s unique role as an easily accessible healthcare provider, they could be at the center of the expansion of MTM programs.
- In 2016, 85% of Medicare Part D plans have a preferred pharmacy network (drugchannels.net)
Centers for Medicaid & Medicare Services just released data on 2016's Medicare Part D plans…analysis reveals that 85% of Medicare Part D regional prescription drug plans will have a preferred cost sharing network in 2016…Preferred network plans are controversial (and generally disliked) by pharmacy owners. That’s because reduced pharmacy profits are the biggest source of cost savings from these networks. Despite ongoing complaints, it's still full speed ahead for preferred networks.
- 5 ways OIG checks hospital safety (healthcareitnews.com)
New this year is quality reporting data…The Office of Inspector General in the Department of Health and Human Services is charged with overseeing the agency's programs to make sure they function efficiently and safely…OIG recently released its work plan for 2016…Here, among the vast responsibilities assigned to the office are five items OIG will check to ensure hospitals provide quality care and maintain safety:
- CMS validation of hospital-submitted quality reporting data
- Hospitals' contingency plans for protecting data in the EHR
- Hospital preparedness and response to high-risk infectious diseases
- Long-term-care hospitals – adverse events in post-acute care for Medicare beneficiaries
- Inpatient rehabilitation facilities – adverse events for Medicare beneficiaries
- Chronic care management: CMS built it, did providers come? (healthcareitnews.com)
On Jan. 1, 2015 hospitals became eligible for reimbursement when treating patients with two or more chronic conditions…Under CPT code 99490, in fact, the Centers for Medicare and Medicaid Services will pay clinicians an average of $43.12 for spending at least 20 minutes in non-face-to-face consults…CMS could pay out as much as $17 billion a year under chronic care management…a surprisingly small number of providers have thus far taken to 99490…While there is a strong appreciation of the benefits of chronic care management, both as fee-for-service revenue...and as a foundation for population health management, providers are struggling to incorporate CCM in their current operations…What's the hold up?...three obstacles: insufficient reimbursement for the time required, lack of awareness about the opportunity, and compliance concerns…the median time spent delivering the service is 35 minutes per patient per month...although non-face-to-face services may be furnished by any qualified clinical staff member, half…are using registered nurses – a more expensive resource than other types of clinical staff – to engage patients.
- Medicare Part D: A First Look at Plan Offerings in 2016 (kff.org)
This issue brief provides an overview of the 2016 PDP marketplace, focusing on key changes from 2015…Many will see higher premiums and deductibles…highlights include:
- beneficiaries in each region will have a choice of 26 PDPs, on average,
- average PDP premium…to increase by 13 percent…from $36.68 to $41.46 per month…largest since 2009
- More than one-third of the 11.2 million PDP enrollees who do not receive Low-Income Subsidies would pay premiums of $60 or more per month…
- Nearly 4.4 million of these enrollees not receiving the LIS face a premium increase of at least $10 per month...
- Two-thirds of all PDPs will have deductibles…a higher share than in previous years. A growing share of PDPs will impose the maximum deductible…to $360..the largest increase in the deductible since the start of the program
- Most PDPs charge coinsurance, rather than flat copayments, for non-preferred brand-name and specialty drugs, which could lead to higher out-of-pocket costs for those who use high-cost drugs.
- Nearly all PDPs use tiered pharmacy networks, with lower cost sharing in selected network pharmacies and higher cost sharing in other network pharmacies, a significant increase
- Beneficiaries receiving the LIS will have access to 7 plans for no monthly premium…fewer than in any past year.
- Pharmacies Face Financial Hardship with Rising DIR Fees (specialtypharmacytimes.com)
Direct and indirect remuneration fees rise from preferred network drug plans offered by insurers and pharmacy benefit managers…fees…are causing financial hardship for many pharmacies…These fees may encompass “pay-to-play” fees for network participation, periodic reimbursement reconciliations, or non-compliance with quality measures…Many pharmacists feel that there is a lack of transparency regarding how DIR fees are calculated…the fees are retroactive, which can make it hard for pharmacy owners to run a business…Originally, DIRs were established to allow PBMs and Medicare to share in rebates that the insurers received from drug manufacturers under Medicare Part D coverage…cost imposed by the PBM on pharmacies that is not necessarily disclosed to Medicare officials…the financial realities associated with very high DIR may force many pharmacies to withdraw from networks…CMS has said in the past that the fees distort the real price of prescription drugs in the market.
- Pharmacy Podcast – Relaunching Medication Therapy Management (pharmacypodcast.com)
Blair Green Thielemier, PharmD…specializes in marketing and implementing new clinical services, such as Medication Therapy Management, in community retail pharmacies. Blair helps to identify opportunities for clinical services, increase workflow efficiency and train pharmacy staff members to offer these services in a sustainable, systematized way…Part D Enhanced Medication Therapy Management model will test methods of optimizing medication use and improving care coordination among Medicare Part D beneficiaries in hopes of finding the most effective ways to attain the objectives of MTM programs. CMS is granting basic, stand-alone prescription drug plans the flexibility to design enhanced MTM programs that include interventions beyond the traditional approach. (podcast 29:19)
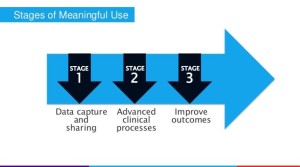
- CMS drops final EHR meaningful use rule (healthcareitnews.com)
Centers for Medicare and Medicaid Services and ONC have released final rules for the EHR Incentive Programs, which they say will ease reporting requirements for providers and allow for 90-day reporting periods…also announced major news on Stage 3 of the program…CMS made some…big changes to the regulations:
- Give providers and state Medicaid agencies 27 months, until Jan. 1, 2018, to comply with the new requirements and prepare for the next set of system improvements.
- Give developers more time to create the next advancements in technology that CMS says will be easier to use and more appropriate to new models of care and access to data by consumers.
- Support provider exchange of health information and interoperable infrastructure for data exchange between providers and with patients.
- Address health information blocking and interoperability between providers.