- CMS issues guidance on AWP compliance (drugstorenews.com)
Centers for Medicare and Medicaid Services…issued guidance to all Medicare Part D plan sponsors regarding the Part D program's "any willing pharmacy" requirement…To comply with the AWP requirement,.. all…plans must have posted standard contracting terms and conditions… This new guidance should help reduce the likelihood of repeating the debacle of early 2015 that affected approximately 400,000 Medicare beneficiaries…
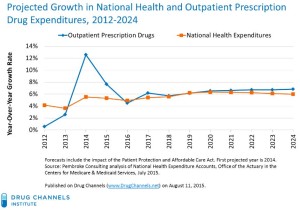
- New CMS Forecast: Drug Spending Grows Along with Impossible Hospital and Doctor Spending (drugchannels.net)
Centers for Medicare & Medicaid Services…released its latest forecasts for national health expenditures...2014, prescription drug spending growth was higher than the growth in total U.S. healthcare spending...the next 10 years,..drug spending is projected to closely track overall healthcare spending. By 2024,.. will spend about $560 billion annually on outpatient prescription drugs...the perceptions that drugs prices are "too high," outpatient prescription drugs will only account for about one of every 10 U.S. healthcare dollars....
- Yelp gets into hospital review business (healthcareitnews.com)
Yelp is teaming up with an unlikely partner to put more healthcare facility data and reviews into the hands of the consumer….announced it would be inking a deal with…ProPublica to integrate data from the Centers for Medicare & Medicaid Services onto the business pages of more than 25,000 healthcare facilities…
- Pending MU rules making hospitals, EHR vendors sweat (healthcareitnews.com)
Until CMS publishes...regulations, all providers and developers can do is hold tight – but the clock keeps ticking...in a real bind....anxiously waiting for the Centers for Medicare & Medicaid Services to release final rule changes to meaningful use regulations...all hospitals and eligible providers would be able to attest to 90 consecutive days of meaningful use for 2015 only, instead of an entire year.
- Additional Reporting May Help CMS Oversee Prescription-Drug Fraud Controls (gao.gov)
GAO found indicators of potential prescription-medication fraud and abuse among thousands of Medicaid beneficiaries and hundreds of prescribers during fiscal year 2011…
- More than 16,000 of the 5.4 million beneficiaries potentially engaged in "doctor shopping,"
- About 700 beneficiaries received more than a 1-year supply of the same drug…
- Recommendation: ..enhance monitoring of potentially wasteful or abusive practices in the Medicaid program, …require states to report to CMS whether their state has lock-in programs for abusers of noncontrolled substances and prohibitions on pharmacy automatic refills, …
- Why Do Pets Have Better Web Portals For Medical Records Than Humans? (healthaffairs.org)
..realities of having children is that it is very likely you will also have pets…with pets come responsibilities…such as...taking your cat to the veterinarian…my vet provides access to a health information web portal for my pets,…it was not until the recent release of proposed revisions to Stage 2 Meaningful Use requirements…that I realized perhaps web portal designers for human electronic health records could learn a thing or two from what I know is already available for pets...
- WEDI makes 4 ICD-10 recommendations to HHS (healthcareitnews.com)
"Without aggressive effort in the time remaining ... lack of readiness may lead to disruption in claims processing."…counting down toward Oct. 1, 2015, the Workgroup for Data Interchange… concern remains… sent a letter to Health and Human Services making four recommendations...
- HHS should expeditiously provide full transparency regarding the readiness of individual Medicaid agencies by state.
- The recently-announced Ombudsman position should be appointed as soon as possible, and WEDI strongly urges CMS to not wait until the compliance deadline to complete this appointment.
- The go-live ICD-10 support plan should include leveraging WEDI's and CMS' implementation support program, which already serves as the central source for collecting ICD-10 (International Classification of Disease) industry issues and solutions.
- Additional outreach is needed in order to help providers with complying with most recent local coverage determination codes.
- Regulators Call for the Revision of Part B Reimbursement Rules for Biosimilars (pharmtech.com)
Centers for Medicare & Medicaid Services issued guidelines on biosimilar reimbursement,.. plans to create a separate code to distinguish a biosimilar from a reference biological, but that any distinguishing identifiers will “have no bearing on coding and payment.” .. the Biosimilars Forum sent a letter to … CMS,.. saying that the proposed rule would limit biosimilar options as a result of reduced investment in their development…
- CMS Suspends Torchmark Part D Plans For ‘Systemic’ Flaws (law360.com)Non-Immediate Suspension of Enrollment and Marketing (cms.gov)
Centers for Medicare & Medicaid Services has suspended enrollment in two Medicare Part D plans sold by Torchmark Corp. because of "widespread and systemic" failures that contributed to the program's highest complaint rate,..."intermediate sanctions" that will result in suspension of enrollment and marketing activities …
- Medicare prescription drug premiums to remain stable (drugstorenews.com)
Centers for Medicare and Medicaid Services…announced that they expect the average premium for a basic Medicare Part D prescription drug plan in 2016 to remain stable at about $32.50 per month.