- Popular heart drugs tainted with carcinogens face a wave of lawsuits (pressherald.com)
The FDA has been coordinating a recall of adulterated heart medications since last July...Dozens of lawsuits have been filed against drug makers and sellers over widely prescribed generic heart medications tainted with potential carcinogens, the first claims in what some lawyers expect to be a wave of litigation...the carcinogen NDMA was discovered in valsartan manufactured by Zhejiang Huahai Pharmaceutical Co. The contaminated valsartan was sold to a number of major drugmakers and used as an ingredient in other popular cardiovascular therapies...Zhejiang Huahai and its affiliates are the primary targets of the lawsuits. Other companies named in the complaints include generic-drug giants Teva Pharmaceutical Industries Ltd. and Mylan NV, as well as CVS Health Corp., which operates large pharmacy and drug-benefit management businesses. Almost 40 defendants have been sued so far...READ MORE
- CMS unveils CAR-T proposal, with emphasis on patient outcomes (biopharmadive.com)
Under a proposal...Medicare would cover CAR-T cell therapies through a Centers for Medicare and Medicaid Services pathway known as Coverage with Evidence Development...The proposal holds a mix of provisions, including that patients must be monitored for at least two years post-treatment. Hospitals administering CAR-T therapy, whether through inpatient or outpatient care, must participate in a CMS-approved registry that collects data on patient outcomes and characteristics and then compares that data to what's been seen in pivotal clinical trials of the therapy or standard of care treatment...Hospitals and clinicians would track certain clinical data elements at baseline, at treatment, and then at three-month, six-month, one-year and two-year follow ups following administration...
- FDA keeps spotlight on GMP data integrity (biopharmadive.com)
The Food and Drug Administration...issued a reminder to drug manufacturers that data integrity remains high on the regulator's agenda of Good Manufacturing Practice-related concerns, publishing new guidance aimed at helping drugmakers meet its standards...The document, which updates a 2016 version, lays out a series of questions and answers for drugmakers explaining how companies can ensure manufacturing data sets are complete, consistent and accurate. While a technical concern, the FDA makes clear that data errors carry real risk to patient health...Guidance isn't the only lever the FDA can pull to help companies comply. In recent years, the agency has flagged data integrity issues in numerous warning letters following inspections and pre-approval assessments.
- FDA unveils open source code for collecting patient data (healthcareitnews.com)FDA’s MyStudies Application (App) (fda.gov)
The Food and Drug Administration launched a new app Wednesday to gather data for clinical trials and other research directly from patients...The FDA released the MyStudies app source code to the public, allowing developers and researchers to tailor the app to suit their research needs. The agency designed the app to facilitate the use of real-world data in research...Patients can submit real-world data to the app via their mobile devices. Researchers can then link those data to other electronic health information. The goal is to improve drug development...“Better capture of real world data, collected from a variety of sources, has the potential to make our new drug development process more efficient, improve safety and help lower the cost of product development,” FDA Commissioner Dr. Scott Gottlieb said in a statement. “Our hope is that the collection of more real world data directly from patients, using a secure app, will lead to more efficient product development and assist with safety monitoring.”
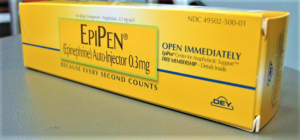
- Pfizer unit Meridian under civil investigation by U.S. Attorney (reuters.com)
Pfizer Inc said...it received a request for documents as part of a U.S. investigation related to quality issues involving the manufacture of auto-injectors at its Meridian Medical Technologies site...Meridian, a unit of Pfizer that manufactures EpiPen injectors...has been hit by a series of manufacturing problems in recent years...In 2017, Meridian had received a warning letter from the Food and Drug Administration. The FDA said Meridian had failed to thoroughly investigate product failures, including EpiPen products that were associated with patient deaths and severe illnesses. It said the company failed to take corrective actions until FDA’s inspection...
- The valsartan carcinogen mess taught pharma a surprise manufacturing lesson. Will 2019 bring more? (fiercepharma.com)
Industry wisdom is that there is nothing new to learn about tablet making. Drugmakers have been doing it essentially the same way for more than 100 years. But that idiom was turned on its head this year when the FDA learned suspected carcinogens could be formed in “sartan” drugs from a specific sequence of manufacturing steps and chemical reactions—and that the U.S. drug supply had been riddled with them for years...The initial discovery of one of the impurities, N-nitrosodimethylamine, came this summer at a U.S. drug manufacturer that had used valsartan API from China’s Zhejiang Huahai Pharmaceutical...the FDA has since learned that the impurities were found in the APIs of other drugmakers, including Aurobindo and Mylan, and in finished products from Sandoz, Teva and others...
- China’s Zhejiang Huahai lambasted in FDA warning letter for putting profits ahead of safety (fiercepharma.com)
The Chinese API maker at the heart of a global scare and recall of blood pressure medicines has been savaged in an FDA warning letter for failing to uncover a suspected carcinogen in its APIs when a customer complained several years ago...The FDA said that when Zhejiang Huahai Pharmaceutical altered its manufacturing process in 2011 to include a solvent suspected of producing the impurity, it didn’t even consider that the changes might lead to the formation of mutagenic impurities in its valsartan APIs...“You failed to adequately assess the potential formation of mutagenic impurities when you implemented the new process,” the highly redacted warning letter says...Regulators in the U.S. and Europe continue to test products to try to ferret out all of the affected drugs. The FDA says there is very little risk of the impurities causing problems, and no adverse reactions have been seen...
- FDA oversight of the prescribing of fentanyl products is inadequate, report finds (healthcarefinancenews.com)Assessment of the FDA Risk Evaluation and Mitigation Strategy for Transmucosal Immediate-Release Fentanyl Products (jamanetwork.com)
The Food and Drug Administration and manufacturers did not take action when evidence emerged that potentially lethal fentanyl products were being inappropriately prescribed to patients, new research...shows...even as evidence emerged that as many as half of patients were taking dangerous medications known as TIRFs that should never have been prescribed to them, the FDA and fentanyl makers did not review prescribing records of even a single physician to consider disqualifying them from the program, which would have prevented them from prescribing the products...The study focused on Transmucosal Immediate-Release Fentanyls, or TIRFs, which are more dangerous than most prescription opioids on the market due to their very high potency and rapid onset. TIRFs are designed to get into the bloodstream within seconds, and because of their risks, were approved by FDA only for adult cancer patients "who are already receiving and who are tolerant to opioid therapy for their underlying persistent cancer pain."
- FDA panel backs prescribing opioid overdose reversal drug along with painkillers (reuters.com)
An advisory panel to the Food and Drug Administration....recommended prescribing the opioid overdose reversal drug, naloxone, along with addictive painkillers...The recommendation of the panel underscores concerns about the growing opioid overdose epidemic...FDA studies found that co-prescribing naloxone to all patients who are prescribed painkillers could increase annual healthcare costs by $63.9 billion to $580.8 billion...I think co-prescribing is an expensive way to saturate the population with naloxone. The at-risk population is not necessarily the ones that are being prescribed new narcotics...
- HHS recommended that the DEA make kratom a Schedule I drug, like LSD or heroin (statnews.com)
The Department of Health and Human Services has recommended a ban on the chemicals in kratom that would make the popular herbal supplement as illegal as heroin or LSD…HHS asserted in a letter to the Drug Enforcement Administration that two chemicals in kratom should be classified as Schedule I substances... FDA...has said that kratom is “an opioid” and has been “associated” with dozens of deaths...Kratom should not be used to treat medical conditions, nor should it be used as an alternative to prescription opioids...Some states have already banned kratom, but it’s currently legal at the federal level. It’s sold in different forms, including dry powder and capsules. According to the American Kratom Association, millions of Americans use the substance.