- FDA to study how drug promotion affects doctors’ decisions (biopharmadive.com)
The Food and Drug Administration is planning to survey 2,000 healthcare professionals to better understand how prescription drug promotion affects the decisions they make...Promotional activities include meetings with pharmaceutical sales representatives, presentations given at industry-sponsored events, and journal or direct mail advertisements. In 2012, drugmakers spent north of $24 billion marketing their products to physicians, according to data cited by the FDA..."Although HCPs (healthcare professionals) are learned intermediaries, like most people, they may rely on heuristics in making decisions and may have cognitive biases in the type of information they attend to at any given time. They may be persuaded by strong statements and may not have the time to ascertain accuracy of such information," the FDA wrote in a notice posted on the Federal Register on March 15..."There is little qualitative research on people’s attitudes to promotion, and this is a major gap," the organization said. "In order to understand people’s perspectives and values more clearly, in‐depth interviews are needed...
- Feds charge 5 doctors over role in alleged Insys bribery scheme (fiercepharma.com)
The case of Insys Therapeutics has played out for several years as suspicions first cropped up in 2014 that the company aggressively marketed its powerful opioid painkiller Subsys, often for off-label uses. Now, the feds have charged five...physicians for taking bribes from the drugmaker in exchange for writing more scripts...In addition to the new complaint, authorities announced that two former Insys employees have taken guilty pleas and are cooperating with the government...doctors Gordon Freedman, Jeffrey Goldstein, Todd Schlifstein, Dialecti Voudouris and Alexandru Burducea face up to 20 years in prison for their alleged participation in the scheme...Allegations against Insys and its former management have been piling up in recent years, and in October, authorities made their way to the company's billionaire founder John Kapoor, charging him with racketeering and other felonies... Prosecutors say the company set up a "speakers bureau" to recruit doctors to write more Subsys scripts, holding "sham" speaking events... the company used the events to funnel money to doctors in exchange for more Subsys scripts, even though many of the prescriptions were outside of the drug's FDA label...
- US FDA track and trace: Industry welcomes data standardisation (in-pharmatechnologist.com)
The US Food and Drug Administration released the Standardization of Data and Documentation Practices for Product Tracing – guidance last week, which elaborates on industry standards relating to the Drug Supply Chain Security Act...(which) aims to build an electronic system to trace prescription medicines in the US...The move to fully electronic traceability information is a positive step in terms of data integrity, availability and exchange in the supply chain...It is important firms within the pharmaceutical supply chain move in the same direction and embrace the advantages of technology and digitisation... Moving to a completely electronic system should only bring advantages to CMOs and the wider industry, improving data integrity and security and reducing the time and resource needed to produce and exchange traceability...
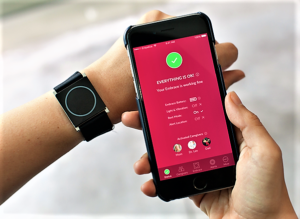
- FDA Clears the First Smart Watch for Use in Neurology (ptcommunity.com)
Wearable device identifies convulsive epileptic seizures and sends alerts to caregivers...The FDA has cleared the Embrace smart watch (Empatica, Inc.) for use by patients with epilepsy. Embrace uses advanced machine learning to monitor for the most dangerous kinds of seizures, known as “grand mal” or “generalized tonic-clonic” seizures, and sends an alert to summon caregivers’ help...The smart watch stands apart from any seizure detection system in that it measures multiple indicators of a seizure. Its unique property is its use of electrodermal activity, a signal used by stress researchers to quantify physiological changes related to sympathetic nervous system activity, also known as the "fight-or-flight" response. Embrace has been approved in Europe as a medical device for seizure monitoring and alert since April 2017.
- FDA issues draft guidance on compounding at outsourcing facilities (biopharmadive.com)
The FDA released a draft version of guidance covering compounding, called "Evaluation of Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Guidance for Industry," directed primarily at outsourcing facilities, addressing the use and qualification of bulk substances in compounding...The proposed rules are an extension of The Drug Quality and Security Act...which identified outsourcing facilities as its own category, separate from traditional compounders...Active pharmaceutical ingredients must be accompanied by a monograph from an appropriate governing party (if a monograph exists), must be made in a facility that has prior approval, and must come with a certificate of analysis (to prove they been characterized)...The agency proposes two specific ways to tell if a compounded drug at an outsourcing facility is safe: whether attributes of the approved drug may make it unsuitable to treat certain patients for particular conditions (including whether the compounded drug is intended to address that attribute), and second, if certain factors for each substance being proposed for use in a compounded drug product – specifically, "its physical and chemical characterization, possible or known safety issues, evidence or lack of thereof of effectiveness, and historical use" — would preclude its use by a third-party facility...The agency says the plan will "clarify and appropriately tailor the policies for traditional compounding pharmacies and the outsourcing facilities that may supply a broader market."...
- Participants in rogue herpes vaccine research take legal action (fiercepharma.com)
Three people injected with an unauthorized herpes vaccine by a Southern Illinois University researcher have filed suit against his company, demanding compensation for alleged adverse side effects from the experiments...SIU professor William Halford, who died in June, had injected Americans with his experimental herpes vaccine...in 2016 and 2013 without safety oversight that is routinely performed by the FDA or an institutional review board...The lawsuit, which was filed Friday in an Illinois circuit court, demands compensation from Halford’s company, Rational Vaccines, alleging his research violated U.S. and international laws aimed at protecting the rights of participants in experiments…Rational Vaccines has said it considers the 2016 trial a success—though it is unclear what data it used to support that claim...SIU has...acknowledged that Halford’s conduct violated university rules and U.S. laws but said that Halford hid his misconduct from the university.
- FDA to Launch New Pilot Program for Orphan Designation Requests (raps.org)
With more than 700 orphan designation requests last year, the Food and Drug Administration...announced a new pilot program to make the request process more efficient...The pilot will include a new form intended to make the submission process easier for sponsors to complete orphan designation requests, and to make the process more efficient for FDA...FDA also released an on-line tutorial to guide sponsors through the submission process and Gottlieb noted there is a new inter-center consult process to streamline and standardize communications...In addition to the pilot, FDA vowed to eliminate the orphan drug designation backlog, and is planning a public meeting to discuss the scientific and regulatory issues related to cancer treatments that target a tumor’s genetic features rather than its location in the body...“We’ll also consider the appropriate application of orphan incentives to this new paradigm of drug development, and how we apply designations to these indications,”...
- Britain’s use of copycat biotech drugs takes off while US lags (reuters.com)
Cut-price copies of an expensive Roche biotech drug for blood cancer have taken 80 percent of the British market since launching last year, saving the healthcare system $113 million a year...The rapid adoption of two so-called biosimilar forms of rituximab from Celltrion and Novartis has been accompanied by discounts of 50 to 60 percent as the National Health Service has used tenders to bring down costs...The situation contrasts sharply with the United States, where regulators have lagged Europe in approving biosimilars while a complex system of rebates offered to insurers by original-brand drugmakers has created barriers to use...The U.S. logjam prompted Food and Drug Administration Commissioner Scott Gottlieb to complain of "rebating mischief" and a "rigged payment scheme"...
- Numbers show drugmakers are keeping RTFs under wraps (biopharmadive.com)
When Celgene Corp. revealed receipt of a Refusal-to-File letter for its blockbuster hopeful multiple sclerosis drug ozanimod, shares in the big biotech dropped as much as 10%...The decline is no surprise — such a major disclosure was bound to hit Celgene's stock price because the Food and Drug Administration's decision not to accept the New Drug Application will significantly impact the time when (and if) ozanimod ever gets to market, potentially costing Celgene hundreds of millions in future sales...But not all pharmas seem to be so upfront with shareholders about the letters from the FDA, according to a BioPharma Dive analysis...Public disclosure therefore falls to the company when it receives one of these rejections. Company executives can disclose as much or as little information about the letters as they deem appropriate...There has been controversy over the issue for years related to CRLs (Complete Response Letters) — and public and investor outcry for the FDA to publish the letters...it comes down to what is "material" to investors, something defined by the Securities and Exchange Commission but which also gives companies some latitude...
- US OKs medical isotope system that isn’t based on bomb-grade uranium (cnbc.com)
The federal government...approved a device made by a private company...that will allow the first domestic production of a medical imaging isotope...a move the government said would enhance national security by reducing the need to transport weapons-grade uranium...The Food and Drug Administration granted the approval to NorthStar Medical Radioisotopes, which said it would begin delivering systems to make technetium-99...the most common isotope in medicine and is used in 40,000 procedures a day in the United States...consumers have long had to depend on a complicated and risky supply chain for the materials...The current process involves shipping weapons-grade, or highly enriched, uranium from the United States to research reactors in Australia, South Africa and Europe where it is irradiated to make molybdenum-99, which decays into technetium-99..."This is a win for our national security," said Peter Hanlon, an official with the National Nuclear Security Administration office of material management and minimization...