- FDA commissioner warns drug companies of ‘disruptive’ regulations to fight opioid epidemic (cnbc.com)
The Food and Drug Administration is likely to take new actions on opioids that may be "disruptive" and "uncomfortable" to drugmakers, the agency's commissioner (Scott Gottlieb) said...In addition to seeking to treat opioid-addicted patients with alternative medications that don't produce a high, the FDA says it will look at ways to reduce exposure to the drug. That includes new ways of packaging and distribution..."For example, it's possible that a defined, short-term supply of medication could be packaged in a manner that limits the number of pills dispensed,"..."We're at a point in this crisis that we're going to have to think of ideas and taking actions that are going to be more disruptive and are going to be uncomfortable to some parties," Gottlieb told "Squawk Box." "But we have to take more vigorous action to get ahead of this."...Gottlieb said the agency is having discussions with drug companies about the new packaging solutions..."Something like this could move potentially quickly," he said. "We're invested in taking a hard look at this and seeing what the opportunities are."...
- U.S. to promote use of opioid alternatives to treat addiction (reuters.com)
The U.S. Food and Drug Administration plans to encourage widespread use among opioid addicts of less harmful opioid drugs such as methadone and buprenorphine, a radical shift in policy that could draw opposition from those in the addiction field who believe abstinence is the only effective treatment...FDA Commissioner Scott Gottlieb outlined a proposal under which every addict who suffers a non-fatal overdose would be treated with an opioid substitute, for long periods if necessary, or even for life...“I know this may make some people uncomfortable,” Gottlieb said...“FDA will join efforts to break the stigma associated with medications used for addiction treatment.”...The FDA, Gottlieb said, will issue guidance for drugmakers to promote the development of new addiction treatments and lay out the agency’s interest in “novel, non-abstinence-based” products...
- What’s new on pharma’s worry list? Cybersecurity, natural disasters, and much more (fiercepharma.com)
Anyone who’s read a Securities and Exchange Commission filing in the U.S. recognizes the risk disclosure section, where biotech and pharma companies list the pitfalls of investing in their shares. Like an investor-oriented version of the risk disclosures in drug ads, they run the gamut from garden-variety competition to political unrest in farflung markets...The accounting and advisory firm BDO sifted through those statements to discover just what the top 100 life sciences companies are most worried about these days.
- Natural disasters, war, conflicts and terrorist attacks...
- The ability to maintain company infrastructure, including IT security and privacy...
- Labor concerns—including pension costs, rising healthcare costs, immigration and outsourcing...
- Litigation. Every company examined listed legal proceedings and lawsuits as risks to their operations...
- Competition and marketing challenges figured in at the top...filings, along with intellectual property protections (or loss thereof) and the success (or not) of current and future drug launches all tied for first place...
- regulation, by FDA and other authorities at the state and federal levels...
- Many drug companies fail to conduct timely safety checks on medicines after FDA approval (reuters.com)
In the rush to approve new medicines, the U.S. Food and Drug Administration often requires drug companies to study possible side effects and alternative doses for medicines once they hit the broader market...A new analysis in the New England Journal of Medicine concludes that, in many cases, that’s not being done...among the 614 studies mandated in 2009 and 2010, 20 percent were never started and 9 percent have been delayed...When drugs are approved, the trials are usually small and short-term, and some side effects may not emerge until the post-marketing phase...The problem is, the faster you get them on the market, the more open questions there are about their safety or the best dose...In some cases, the FDA has simply dropped a requirement for a postapproval study without giving a reason...
- Smartphone-compatible ultrasound device gets FDA nod (pharmaphorum.com)
An iPhone-compatible ultrasound device that can deliver scanning for a fraction of the price of leading technologies, has been approved by the FDA...Traditionally, ultrasound scanners consist of three transducers connecting to large, bulky units which, when combined, carry a hefty price tag...The Butterfly iQ – the world’s first ‘ultrasound-on-a-chip’ device – houses all three of these transducers and over 10,000 sensors in a single handheld scanner, allowing for faster, easier, and cheaper ultrasound scanning...Doctors perform an ultrasound scan as they usually would with imagery appearing on their smartphone. The images are then sent to cloud storage, allowing for connectivity to hospital medical record systems...Offering a unique blend of affordability, diagnostic versatility, and assistive intelligence, Butterfly has the potential to impact human health more profoundly than any diagnostic device since the stethoscope, invented over 200 years ago. At less than $2,000, healthcare providers can purchase an easy-to-use, powerful, whole-body medical imaging system that fits in their pocket...
- Navigating New FDA Guidance on Preapproval Payer (ajmc.com)
In light of recent guidance from the FDA on appropriate communications between payers and drug manufacturers prior to a drug’s approval, a panel of stakeholders at the Academy of Managed Care Pharmacy 2017 Nexus meeting...discussed how these new guidelines can raise as many questions as they answer.
Soumi Saha...background on the implications of the guidance...Payers and other healthcare decision makers need thorough healthcare economic information (HCEI) on a potential new drug’s budgetary impact and treatment population in order to plan and budget their resources...Saha pointed to surveys that found that 64% of payers perceived a gap between the information they needed and what they received, and 91% of manufacturers said it was difficult to have their HCEI materials approved under FDAM (Food and Drug Administration Modernization Act)...
Mark Gaydos...was not aware of any enforcement letters that had been sent by the FDA regarding formulary communications, and that it would have been difficult for the FDA’s Office of Prescription Drug Promotion to write such letters anyway, considering the lack of clarity in the rules...
Jay Jackson...provided more insight on how manufacturers are using the updated guidance to change how they generate HCEI. It is important for companies to establish standards on the scope of the data they will present, the scientific evidence supporting it, and how it should be presented, in a process he called “beginning with the end in mind.”...“I think we’re moving in the right direction,” Jackson said. “We’re not even 1 year into this, but I do think there’s increased knowledge of how to handle” HCEI both proactively and reactively....
Kat Wolf Khatchatourian...discussed her experiences with HCEI communications...Because formularies and rate filings are locked in by June for the next year, they cannot be adjusted to account for any additional products that are approved later on in the year. As such, plans are “perpetually playing catch-up with the innovation that’s coming to market.”...She called for additional legislation that “would improve both the timing and the quality of information that’s able to be exchanged and enable a safe harbor where people are not fearful” of sharing information...
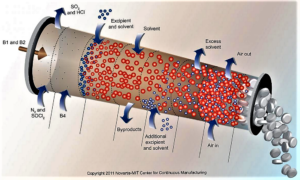
- Continuous Manufacturing: Pfizer, Vertex, AstraZeneca and Others Weigh FDA Plans (raps.org)
The US Food and Drug Administration has been encouraging the adoption of continuous manufacturing techniques...and several companies recently offered the agency some suggestions to refine its work around the developing technology...continuous manufacturing allows companies to move more seamlessly and efficiently...Last week, FDA finalized guidance on how manufacturers can participate in the agency’s program to advance continuous manufacturing...And in a blog post, Michael Kopcha, director of FDA's Office of Pharmaceutical Quality, pointed to Vertex's cystic fibrosis drug Orkambi (lumacaftor/ivacaftor) and Janssen's HIV treatment Prezista (darunavir) as examples of companies successfully using continuous manufacturing after engaging with FDA's emerging technology team...Vertex Pharmaceuticals...noted several contradictions in how batch size is described in an FDA document and sought further clarity and certainty regarding FDA’s understanding and expectations regarding batch size...AstraZeneca...asked if FDA might consider harmonizing the assessment process of the changes relating to continuous manufacturing, including a mechanism by which there could be some mutual recognition across countries participating in the International Council on Harmonization. AstraZeneca also said it "does not see the need" for a full ICH guideline on continuous manufacturing, which the company says FDA has been supporting. But the European drug industry group known as EFPIA is suggesting a Q&A document based on ICH Q8 and AstraZeneca says it "concurs with that approach."
- Is FDA Going Too Far in Enforcing Drug Compounding Law? (c.ymcdn.com)
...the FDA is overstepping its authority and jeopardizing patient access to compounded medications...The pharmacies say the Food and Drug Administration has misinterpreted Congress’s intent when it enacted the Drug Quality and Security Act (DQSA)...by not allowing office-use compounding...The pharmacies also are concerned that the FDA has issued no rules for compounding but only guidance documents, which aren’t legally enforceable...‘Mass confusion has resulted from the usage of guidance documents, and the FDA is definitely circumventing the Administrative Procedure Act (APA) in our opinion,’’ Cynthia Blankenship, executive vice president of the International Academy of Compounding Pharmacists (IACP)...The APA governs how federal agencies propose and establish regulations. IACP wants Congress to pass a bill (H.R. 2871) they say will fix these issues...Since the DQSA was enacted, the FDA has significantly..increased its inspections of drug compounding...facilities...The FDA has conducted more than 350 inspections of compounding pharmacies as of Nov. 27, 2016, and nearly 120 of those inspections were due to reports of serious adverse events or product quality issues...
- Shortages of drugs and saline reported as Puerto Rico hurricane damage lingers (fiercepharma.com)
Shortages of drugs and saline produced in Puerto Rico are beginning to materialize after Hurricanes Irma and Maria wreaked havoc on production on the island, which produces about 10% of the U.S. drug supply including products like Lipitor and blood thinner Xarelto...Saline solution was already suffering supply restraints before the storms knocked out power to plants across island, affecting saline production at a facility operated by Baxter International...The company, which has said it lost days of production as a result of the storms, has put customers on allocation of sodium chloride and will try to make up for some of that supply by importing saline and glucose from plants in Australia and Ireland...The FDA has said that there are about 40 drugs manufactured in Puerto Rico, 13 of them exclusively, and that shortages of some of those will be materializing within days. The storms knocked out power, and while manufacturers have backup generators, they could be without commercial power for months. Most of the facilities that have resumed production, maintain only partial operations…
- FDA warns ‘critical’ drug shortages possible after Hurricane Maria battered Puerto Rico (usatoday.com)
Patients could experience "critical shortages" of key pharmaceuticals, the U.S. Food and Drug Administration is warning after Hurricane Maria brought Puerto Rico's drug manufacturing industry to a standstill...The FDA said...it is taking active measures to help redirect production and preserve existing treatments to avoid a ballooning health crisis from Maria's destruction...The agency did not identify any specific medications that could be at risk of a shortfall, and a spokesperson was not immediately available to provide details...But there are "several" cases where "we may soon face critical shortages if we don’t find a path for removal or ways to get production back up and running," FDA Commissioner Scott Gottlieb said in a statement...Some companies are beginning to move product off of the island, and they’ve been communicating with the FDA about that and what potential challenges and limiting factors they see ahead...Drugs made on the island include AstraZeneca's cholesterol treatment Crestor, Abbvie arthritis drug Humira and Johnson & Johnson-owned HIV drug Prezista. Those three companies have said supplies of their drugs are in good shape...the catastrophic storm wiped out electricity for the entire island, devastated telecommunications and made travel nearly impossible for many employees of the island's nearly 50 pharmaceutical factories...