- Heated And Deep-Pocketed Battle Erupts Over 340B Drug Discount Program (khn.org)
A 25-year-old federal drug discount program has grown so big and controversial that it faces a fight for survival as federal officials and lawmakers furiously debate the program’s reach...The program, known as 340B, requires pharmaceutical companies to give steep discounts to hospitals and clinics that serve high volumes of low-income patients...The Centers for Medicare & Medicaid Services...cut Medicare payments for hospitals enrolled in the program by 28 percent...About 40 percent of the hospitals in the U.S. now buy drugs through the program...the hospital lobbying group 340B Health, said that for some small, rural hospitals the funding cut “could actually be the difference between staying open and closing.”...those supporting the cut, including drugmakers, argue that the program has grown beyond its original intent because hospitals have pocketed the discounts to pad profits — not to help indigent patients...Stephen Ubl, president of drug industry group PhRMA, said the program “needs fundamental reform” and that the latest rule change is merely a good first step. His group, which has deep pockets and an advertising campaign geared at pinpointing the program’s flaws, has a list of changes that Congress and the Trump administration could tackle. Those include limiting which hospitals should be eligible for 340B price breaks and making sure needy patients benefit when hospitals buy discounted drugs.
- Nevadans slow to embrace state’s health insurance exchange, data show (reviewjournal.com)
New numbers show Nevadans have been slow to jump into the state's health insurance exchange…Department of Health and Human Services reported Wednesday that 23,248 Nevadans bought coverage through Nevada Health Link in the first month of open enrollment, from Nov. 1 through Saturday…That's roughly half of the 40,285 residents who bought a plan through the exchange in the first month of sign-ups a year ago…Federal officials said enrollment should pick up as the Dec. 15 deadline nears to buy a plan with a Jan. 1 start date...Consumers without coverage in place by then face a federal tax for going without health insurance.
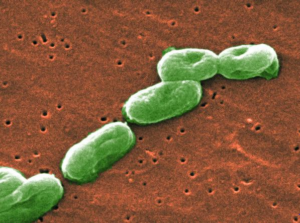
- Feds blame multistate B. cepacia outbreak on PharmaTech plant’s water system (fiercepharma.com)
After a months-long investigation, federal officials have nailed down the source of a Burkholderia cepacia outbreak that made its way into several states and infected dozens of patients...the Centers for Disease Control and Prevention and the FDA said they had detected the B. cepacia bacteria in the water system at Florida-based CMO PharmaTech. The company had produced 10 contaminated lots of constipation drug docusate sodium before 6 distributors shipped the meds around the country. In total, 60 people in 8 states became infected...The outbreak started in late June in ventilated cystic fibrosis patients. During the investigation, CDC officials said infections could be life-threatening in patients with compromised immune systems or lung conditions.
- Ohio prisons officials challenge FDA stand on execution drug (washingtonpost.com)
With two dozen scheduled executions in limbo, Ohio sent a forceful letter to Washington… asserting that the state believes it can obtain a lethal-injection drug from overseas without violating any laws…stopped short of suggesting Ohio is moving forward to obtain the powerful sedative sodium thiopental…the state asked to begin discussing with federal officials about acquiring the substance legally…FDA had warned Ohio in June that importing the restricted drug could be illegal…setting up the latest roadblock that Ohio and several other states have faced in carrying out the death penalty…States have struggled to obtain lethal injection drugs since pharmaceutical companies discontinued the medications they traditionally used or put them off limits for executions…"My sense is that the Food and Drug Administration…was never designed to create an additional impediment to states trying to carry out lawful sentences,"…
- The untold story of TV’s first prescription drug ad (statnews.com)
On May 19, 1983, Boots aired the first broadcast television commercial in the United States for a prescription drug, the pain reliever Rufen...Within 48 hours of the ad’s airing, the federal government told the company to take it down. And more than 30 years later, the fight over marketing prescription drugs directly to the public is still raging...Now, the American Medical Association, the largest doctors group in the United States, wants to stop direct-to-consumer advertising for prescription drugs in the belief that the ads encourage patients to seek medicines unnecessarily. But the effort to have drug ads banned alongside tobacco ads will face plenty of obstacles, none bigger than the First Amendment. Perhaps the most unusual thing about this decades-long saga is that it’s an issue at all...The United States is one of only two countries in the world to allow these ads. How did this little-noted example of American exceptionalism come to be?...It started with Boots.