- 8 Things to Know About Biosimilars (medscape.com)
With the recent US Food and Drug Administration approval of a fourth biosimilar medication, these compounds remain a hot topic in many areas of medicine, including nephrology, oncology, endocrinology, gastroenterology, and rheumatology…Despite the growing body of knowledge about biosimilars—and despite their increasing coverage in peer-reviewed journals and presentations—a recent Biosimilars Forum press release reveals that more physician education is needed about these medications...
- What Are Biosimilar Medications?
- What Is the Approval Process of Biosimilars?
- How Many Are Approved, and for What Conditions?
- What Does 'Interchangeable' Mean?
- What Is Extrapolation?
- Are Biosimilars Safe, and Can They Be Trusted?
- Do Patients Know and Trust Biosimilars?
- How Do the Costs of Biosimilars Compare to Biologics?
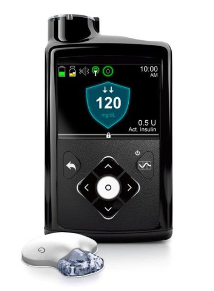
- FDA approves 1st ‘artificial pancreas’ for type 1 diabetes (upi.com)
The Food and Drug Administration...approved the first automated insulin delivery system -- a so-called "artificial pancreas" -- for people with type 1 diabetes...This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin…The device -- Medtronic's MiniMed 670G -- is what's known as a hybrid closed-loop system. That means it monitors blood sugar and then delivers necessary background insulin doses. The device will also shut off when blood sugar levels drop too low...this device isn't yet a fully automated artificial pancreas. People with type 1 diabetes will still need to figure out how many carbohydrates are in their food, and enter that information into the system, the agency noted..
- FDA criminal office draws fire from agents and doctors over drug import crackdown (reuters.com)
The FDA’s Office of Criminal Investigations has spent thousands of hours pursuing foreign-imported, mislabeled drugs. But more than half of all OCI cases end without charges, and critics contend the agency’s efforts protect drug makers as much as consumers...On April 5, 2012, a criminal investigator from the Food and Drug Administration named Robert West charged into an oncology clinic...West was chasing a lead that Dr. Anindya Sen...purchased an unapproved...cancer drug Avastin...Without a warrant or permission, he and an FBI agent rifled through cabinets, seizing drugs that appeared to have foreign, non-FDA approved packaging…A...judge later said West’s...statement about the drugs being counterfeit "apparently was not the truth." West’s search was declared illegal, and the evidence was deemed inadmissible…Prosecutors are declining to pursue many FDA cases, citing a lack of prosecutorial merit, criminal intent or strong evidence, Reuters found in a review of more than 170 letters detailing why the Department of Justice declined cases. The letters, obtained under the Freedom of Information Act, appear to bolster critics’ claims of agency overreaching…
- The dark side of ‘compassionate use’ of experimental drugs (washingtonpost.com)To help cancer patients, lawmakers pushed access to a controversial doctor (statnews.com)
This is a picture of Josh Hardy...Josh...had undergone a bone-marrow transplant for kidney cancer...He was dying...There was an experimental drug called brincidofovir...his doctors thought might work. But the company declined their repeated requests to provide it. His parents...rallied friends, who rallied their friends and their friends...until it seemed as though the entire Internet were behind them...The ending was a good one: The company gave Josh the drug, and it worked and he got to go home...But not all cases like this go so well. A powerful report from STAT this week provides a heartbreaking reminder that the reason experimental drugs are not available for anyone to use is because they are just that — experimental. And the chances that things will go wrong are as strong as that they will go right...
- Juno says two more patients die in leukemia drug trial (reuters.com)
Juno Therapeutics Inc said two more patients had died after suffering brain swelling during a trial of its experimental genetically engineered leukemia drug, bringing the total up to five...Juno said...it had voluntarily put the mid-stage study on hold and informed the U.S. Food and Drug Administration earlier this week...The company is still evaluating the cause of the deaths and has not yet decided whether it will continue developing the drug...JCAR015 is an experimental chimeric antigen receptor T-Cell therapy...The FDA, which had imposed a hold on the trial after the first three deaths, lifted it soon after Juno agreed to revert to its original drug regimen that excluded fludarabine...The elimination of fludarabine reduced toxicity but has not proved to be the only contributing factor...
- Behind the Sarepta drug approval was intense FDA bickering (statnews.com)
The run-up to Monday’s approval of a Sarepta Therapeutics drug to treat Duchenne muscular dystrophy was marked by unusual bickering inside the Food and Drug Administration, where debate over a key scientific question morphed into a formal dispute, and the head of the drug review division was accused of being too intensely involved in the process for evaluating the medicine...the decision to greenlight the drug fell to the FDA Commissioner, Dr. Robert Califf...he deferred to Dr. Janet Woodcock, the controversial head of the drug review division, who pushed hard to approve the Sarepta medication but clashed with other FDA officials along the way...This has been a highly charged issue and has transformed the Sarepta drug into a litmus test for agency approval of new medicines, notably for diseases with unmet medical needs. Seen through that prism...approving the drug would be detrimental to the FDA approval process on a long-term basis.
- FDA slams drug maker for touting unapproved leukemia treatment to docs (statnews.com)
Celator Pharmaceuticals proudly displayed a large poster touting its experimental Vyxeos (CPX-351) medication as an effective salve for treating acute myeloid leukemia. The poster was, in fact, one of countless placards featured prominently on the exhibit floor at the American Society of Clinical Oncology meeting…the Celator poster managed to stick out...Vyxeos has not yet been approved to treat AML...Celator reported positive clinical trial results for the drug...But that’s not the same thing as having clearance to market a product. Unfortunately, that’s the impression regulators had after looking at the poster…As far as the Food and Drug Administration is concerned, Celator was, effectively, promoting a drug that was misbranded. And so the FDA sent the company (now Jazz Pharmaceuticals)...a letter to complain about its promotional behavior...From a public health perspective, these claims and presentations are concerning because they include representations in a promotional context regarding the safety and efficacy of an investigational new drug that has not been approved by the FDA...
- FDA issues guidelines for female libido pills after learning some hard lessons (statnews.com)
After a two-year wait, the US Food and Drug Administration finally issued new guidance for companies that want to develop drugs to bolster female libidos. But the details suggest the agency has belatedly learned some hard-fought lessons following complaints that the controversial Addyi pill did not warrant approval last year...The 15-page draft guidance...offers a typical how-to for companies, but also points to certain steps that Sprout Pharmaceuticals did not follow as part of its Addyi marketing application...The drug, which is now sold by Valeant Pharmaceuticals, was approved despite debate over its safety and effectiveness, and the extent to which medicines should be used to treat female sexual dysfunction...the FDA is locking the barn door after the horse got out. So now, the agency is telling other companies to do some things that Sprout didn’t do...they’ve made it harder to get a drug approved but they have extended helping hands in numerous places...unless [a company] really looks hard for subgroups [of patients], the whole effort is hopeless...
- Compounding Pharmacies: Safety Blind Spot (morningconsult.com)
To protect patients, pharmaceutical manufacturers must monitor any adverse events we hear about with respect to the drugs we produce and report those adverse events to the Food and Drug Administration, as required by law...Developing and maintaining an accurate safety profile of a product is a joint responsibility between the manufacturer of the product, the FDA and consumers...As noted, reporting of adverse events to the FDA is a critical component of the patient safety protection system...While some compounding pharmacy adverse events are now haphazardly reported — FDA just last week sent warning letters to drug compounding pharmacies in Tennessee and Virginia following reports — there is a giant gap in that system that may put patients at risk —adverse events with respect to drugs made by compounding pharmacies are generally not required to be reported to anyone...Regardless of how quickly states work to improve their oversight of sterile compounding, an immediate step should be to require reporting of adverse events. If states do not act to require this kind of reporting, Congress should step in and mandate it. Until then, patient safety is at risk.
- China Drug Sales to the U.S. Grow Despite Safety Concerns at Home (bloomberg.com)
Chinese drugs and pharmaceutical ingredients are found in medicine cabinets as far away as New York and Chicago, and the country’s exports of pharmaceutical products and health supplements worldwide jumped 3 percent to $56 billion last year...Yet even as China’s drug industry has grown in global stature, so have questions about the safety of its products...about 700 Chinese firms were told by regulators in China to review their pending applications to sell new drugs and voluntarily withdraw any that were false or incomplete. Within months, about 75 percent had been retracted by the manufacturers or rejected by Chinese officials...Among those were some medicines that were separately approved for sale in the U.S. by the Food and Drug Administration. Some of the companies say their data in China were flawed because of faulty information by local research firms...