- Drugmakers can’t charge beyond making costs for compassionate use: FDA (reuters.com)
Food and Drug Administration said companies could only charge patients for the cost of manufacturing experimental treatments used under compassionate grounds, and it cannot force government or private health insurers to pay for these drugs..."Compassionate use" of experimental drugs allows physicians to prescribe unapproved treatments for patients who have no other satisfactory alternatives in the market...The FDA's move seems to be intended to soften the repercussions of its possible rejection of Sarepta Therapeutics Inc's muscle-wasting drug (Duchenne muscular dystrophy)...FDA last week deferred its decision on whether to approve Sarepta's drug, eteplirsen, after an advisory panel determined that the treatment was not effective...The FDA is trying to create a compromise, saying drug companies can charge for a drug even if its not approved...Sarepta's drug has been in the spotlight over the past few months with patient groups and parents arguing passionately in favor of the treatment to pressure the regulator to approve the drug.
- FDA wants drug makers to pay fees to review OTC medicines (statnews.com)
The US Food and Drug Administration is paid fees by the pharmaceutical industry to review brand-name and generic drugs. Now, the agency wants to collect money to review over-the-counter medicines...Specifically, the agency wants companies to pay for reviewing OTC monographs. Unlike brand-name and generic drugs, which involve individual companies seeking approval to market individual drugs, the OTC monographs refer to multiple medicines that share the same ingredient for the same use. Moreover, numerous companies can make these drugs, but do not require FDA approval prior to marketing...the pharmaceutical industry has largely supported so-called user fees...the fees helped transform the relationship between companies and regulators, who are under increasing pressure to meet benchmarks for speeding applications through the review process...Given the workload, the FDA argued that user fees will only bolster its ability to review OTC medicines…the large OTC companies interested in a modernized system who will support it in exchange for some concessions from FDA...The smaller, more fragmented (companies) will probably oppose it as (fees represent) more regulatory burden on them in an area that has worked for decades without fees
- FDA reconsiders training requirements for painkillers (hosted.ap.org)
Food and Drug Administration is reconsidering whether doctors who prescribe painkillers like OxyContin should be required to take safety training courses...The review comes as regulators disclosed that the number of doctors who completed voluntary training programs is less than half that targeted by the agency...Under the current risk-management programs, drugmakers fund voluntary training for physicians on how to safely prescribe their medications...many experts - including a previous panel of FDA advisers - said those measures don't go far enough and that physician training should be mandatory...The FDA's initial ideas to improve safety included mandatory certification for doctors and a national registry to track patients taking the drugs. But industry pushed back. Drugmakers and the pain specialty groups they fund argued that certification would be too burdensome for doctors, leaving many patients undertreated. And patient groups said that registries would unfairly stigmatize those who rely on painkillers to deal with long-term pain....
- Proposed legislation could heighten controversy over compounding pharmacies (statnews.com)FDA Issues Three New Draft Guidances (iacprx.org)
A congressional committee is proposing an amendment to a spending bill that may intensify the debate over the safety of compounded medicines...The House Appropriations Committee has introduced language that would alter a key requirement for pharmacists to make and dispense compounded medicines, which are generally customized for specific patient needs. And the language runs counter to a draft guidance that the US Food and Drug Administration released...that would govern compounding practices...Specifically, the amendment would allow pharmacists to compound medicines without needing prescriptions for individual patients. This has been a contentious issue in the wake of the 2012 outbreak of fungal meningitis that was tied to a compounding pharmacy and led to 64 deaths. The episode underscored confusion between federal and state oversight of compounders...the Drug Quality and Security Act went into effect to sort out enforcement authority...it...created two classes of compounders — traditional compounders who make medicines for individual patients, and others who resemble drug makers by making large amounts of a drug. These compounders must register with the FDA and are subject to greater federal oversight...The Pew Charitable Trusts...argued that the language proposed by the House committee would blur that distinction. And the nonprofit contended the change could jeopardize public safety...International Academy of Compounding Pharmacists..."The FDA continues to ignore clear congressional intent where the states have clearly indicated pathways in which pharmacies can participate in office-use compounding. The 2017 appropriations bill reinforces this intent."..."FDA’s draft guidance on anticipatory compounding is confusing at best and may ignore the clear language of bill when it comes to defining this practice,"…
- Can a pricey implant to treat opioid addiction save lives — and money? (statnews.com)
The implant promises to treat opioid addiction without the hassle of a daily pill. And the company marketing the drug is so confident it’ll work, it’s planning to offer insurers a twist on a money-back guarantee: If the new device doesn’t save them money, they’ll get a refund...The implant, branded as Probuphine, relies on four tiny rods implanted under the skin to dispense the drug buprenorphine for six months at a time. The Food and Drug Administration is expected to decide on Friday whether to approve it...Braeburn Pharmaceuticals...has commercial rights to the implant...plans to price the implant "competitively" with other injectable drugs for neuropsychological conditions…"We are going to put our money where our mouth is," said Braeburn CEO Behshad Sheldon. "We believe that when you guarantee compliance with a medicine, it is going to save money in the long run."...The FDA rejected the implant the first time it came before the agency in 2013, requesting more data demonstrating its efficacy.
- Public wary of faster approvals of new drugs, STAT-Harvard poll finds (statnews.com)
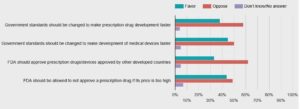
A majority of Americans opposes federal regulatory changes to speed up the development and approval of new medical treatments, a new STAT-Harvard poll finds — suggesting the public has serious doubts about legislation now moving through Congress...both parties are pushing to change government regulatory standards that they blame for slowing the approval process to get new products to patients...nearly 6 out of 10 Americans said they oppose changing government safety and effectiveness standards to allow for faster approvals of new prescription drugs by the Food and Drug Administration, while 38 percent said they’re in favor of speedier FDA action...The poll sheds new light on how Americans balance their priorities between speed and safety in the approval of new medical treatments.
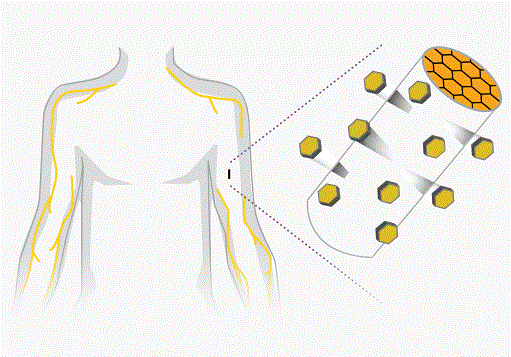
- New implant set to join fight against U.S. painkiller epidemic (reuters.com)
Two companies are on the cusp of taking a new treatment for opioid addiction to the U.S. market at a time when lawmakers are seeking ways to arrest an epidemic of heroin and painkiller abuse...Titan Pharmaceuticals Inc and...Braeburn Pharmaceuticals have together developed a matchstick-sized implant that analysts expect will be approved next month, despite mixed reviews...Implanted into the arm, the treatment is designed to be less vulnerable to abuse or illicit resale than the oral drugs that are currently used to treat opioid addiction...Two drugs are predominantly used to treat opioid addiction: methadone, which is dispensed only in government-endorsed clinics, and the less-addictive buprenorphine, which exists as a pill or strip of film...The implant, known as Probuphine, offers an alternative by administering buprenorphine for up to six months after users have first been stabilized on the oral form of the drug...Food and Drug Administration have raised reservations about possible complications from the insertion and removal of the 26mm long implant...
- Experts Decry Tying Medical Research Funds to FDA Standards Changes (wsj.com)
Moves in Congress to link billions of dollars in new medical research funding to revised standards for drug and medical-device approvals are troubling some public-health experts, who say the combination makes it too easy for lawmakers to support lower patient-safety standards...These safety advocates say legislation to beef up research funding for the National Institutes of Health should be separated from product-approval changes at the Food and Drug Administration..."This is the first time this has been done this way, and it’s a deal with the devil," said Dr. David A. Kessler, onetime FDA commissioner during the 1990s under presidents of both parties. "It’s time to uncouple the promise of research funding from the requirement that FDA standards be lowered."...To its supporters, linking NIH funding with FDA bills (21st Century Cures Act)—including changes to approvals of antibiotics and some medical devices—produces innovation in life-saving products and research. Politically, it is a classic Washington bargain that has something for everyone...The new FDA commissioner, Dr. Robert M. Califf, said in a recent talk that "this legislation, if not carefully crafted, could pose significant risks for FDA and American patients…Innovative therapies are not helpful to patients if they don’t work, or worse, cause harm," he said...
- FDA Approves New Version of Oxycodone (painnewsnetwork.org)
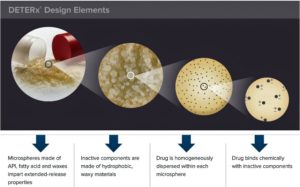
Food and Drug Administration has approved a new extended released version of the opioid painkiller oxycodone that has abuse deterrent properties unlike anything else on the market...Xtampza – can be ingested in capsule form, but users can also sprinkle the capsule contents on soft foods or into a cup, and then directly into the mouth...The medication, which can also be ingested through a feeding tube, is the sixth opioid pain medication with an abuse deterrent formula to be approved by the FDA...Xtampza is made by...Collegium Pharmaceutical with proprietary technology (DETERx technology platform) that combines oxycodone with fatty acid and waxes to form small spherical beads that are placed inside the capsule...The beads are designed to resist breaking, crushing, chewing, dissolving and melting, methods long used by drugs abusers to snort or inject opioids.
- China Eases Path for Foreign Drugmakers’ Hepatitis C Treatments (nasdaq.com)
China will grant four global drug companies priority-review status to launch groundbreaking new hepatitis C treatments in China, a rare move to open the lucrative market to foreign players...China's Food and Drug Administration expedites domestic drug applications to encourage innovation. But its lengthy drug-approval process for foreign companies means none of the direct-acting antiviral agents that have been shown to cure more than 90% of hepatitis C patients within a few months have been approved in China, which has among the highest rates of the disease in the world with an estimated 10 million people infected...It shows that the CFDA is serious about prioritizing important new innovative medicines that address real unmet medical need or improve substantially on what's currently available, whether they originate in domestic or [global] pharma companies...The policy also encourages foreign companies to manufacture drugs in China, saying companies will qualify for priority treatment if they submit applications for approvals in China simultaneously with U.S. and European Union approvals and use the same production standards as in those markets.