- VA expands hepatitis C treatment to all patients with the virus (militarytimes.com)
Veterans Affairs Department will begin providing hepatitis C treatment to all veterans in its health system who have the virus, regardless of their disease stage...Having received a boost in funding from Congress late last year for the costly medications needed to cure hepatitis C, the VA is now able to treat the 174,000 veterans in its health system who have the disease…The Food and Drug Administration in January approved Zepatier (elbasvir and grazoprevir), made by Merck, to treat the disease...executives said they priced the medication to broaden and accelerate access to treatment for patients covered in commercial or public plans, including our country’s veterans...This is a good example of how government and industry can work together toward a shared goal in the best interests of public health — particularly for our veterans who are so deserving...Merck spokeswoman said it was too early to tell whether Zepatier will become the favored treatment within VA...but that the company priced it appropriately to ensure that it could be accessed by all veterans.
- France raises concerns about heparin made by Dongying Tiandong (in-pharmatechnologist.com)
French regulators have raised concerns about heparin made by Chinese supplier Dongying Tiandong Pharmaceutical and called on the EMA to revoke its GMP certificate...a report filed on the European Medicines Agency’s EudraGMP database last week , ANSM inspectors who visited the firm’s facility in Dongying City in December observed 10 deviations from good manufacturing practices standards...The ANSM inspectors said Dongying had used seven batches of out of specification crude heparin to make active pharmaceutical ingredients between 2014 and 2015, which they said indicated a misunderstanding of the basic GMP principles...According to Dongying's website, the firm passed a US Food and Drug Administration inspection in 2013 and was been cleared to supply heparin sodium and enoxaparin sodium APIs by Mexican authorities in 2014.
- Health Officials Urge FDA To Add Black Box Warning On Opioids, Benzos (forbes.com)
City and health directors from across the country are urging the Food and Drug Administration to adopt new labels to "explicitly warn about a dangerous combination of medications" that have been fueling the nation’s prescription drug overdose epidemic over the years...Available online, the petition, which has been signed by academics, researchers and physicians, requests that the FDA amend black box warnings on all opioid analgesic and benzodiazepine class medications...to require medication guides that specifically warn patients of concurrently using both drug classes...We believe that this black box warning is a critical first step in raising awareness around the danger of co-prescribing these medications…The warning educates doctors so that they can provide the highest quality of care to patients...
- FDA should warn of risks of opioid, benzo combo, say public health experts (statnews.com)CITIZEN PETITION (health.baltimorecity.gov)Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study (bmj.com)
Public health officials around the country want the Food and Drug Administration to warn people about the risks of taking opioids along with common anti-anxiety drugs...Forty-one public health officials and researchers signed a petition, which will be submitted to the FDA... It calls for so-called black box warnings on both opioids and benzodiazepines, indicating that their concurrent use "contributes to the risk of fatal overdose." Opioids are intended primarily for pain relief; benzodiazepines, like Valium and Xanax, are tranquilizers that can treat anxiety...A 2015 study in the British Medical Journal found that US veterans taking opioids who took more benzodiazepines were more likely to die than those who took fewer benzodiazepines...Deaths from both opioids and benzodiazepines have been on the rise...
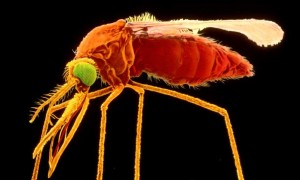
- Intrexon says FDA finds anti-Zika mosquito environmentally safe (reuters.com)FDA Publishes Preliminary Finding of No Significant Impact on Oxitec’s Self-limiting Mosquito (oxitec.com)
A genetically engineered mosquito being used in the fight against Zika will not have a significant impact on the environment, the maker Intrexon Corp said, citing preliminary findings from the U.S. Food and Drug Administration...Males of the self-limiting strain of the Aedes aegypti mosquito are modified so their offspring die before being able to reproduce, says Intrexon...The FDA findings agree with the draft environmental assessment submitted by Oxitec, the UK unit of Intrexon that developed the mosquito.
- FDA still struggling with backlog of generic drug applications (statnews.com)
Under pressure to speed approval of generic medicines, the Food and Drug Administration...released data to defend its progress...the statistics indicate the agency is making headway, there are also clear signs the FDA continues to struggle with the workload...the number of full and tentative drug approvals has been rising each month since last April and reached 99 this past December...the agency also appears to be doing a better job of communicating with generic drug makers about their applications...Generic drug approval is gaining more attention thanks to the intensifying national debate over the rising cost of prescription medicines. Although prices have also risen for some of these copycat medicines, generics remain...lower-cost alternatives to brand-name drugs. And generics now account for 88 percent of all prescriptions written...The FDA is being a little disingenuous saying its backlog is almost cleared...The FDA faces...the increasingly large number of applications that drug makers are submitting...more than 4,000 have been filed in the past four years...the FDA workload will not abate...The upshot is that the rate at which new generics will find their way to pharmacy shelves is unclear — and that adds further uncertainty for health care budgets...
- Swedish industry wants European medicines agency if UK quits EU (reuters.com)EFPIA Statement on Brexit (efpia.eu)
Sweden should become the new home of the European Medicines Agency if Britons vote to leave the European Union in a June referendum, according to the head of the Swedish pharmaceutical association...The agency, which approves medicines for all EU countries, has been based in London since it started in 1995. However, a so-called Brexit would leave Europe's equivalent of the U.S. Food and Drug Administration outside the bloc and could force a move...Shifting to Sweden would make sense, given the country's scientific strength and the leading role Swedish experts already play in European drug regulation…If the referendum in the UK results in a 'no' to the EU, the government should immediately launch an intensive lobbying campaign to make Sweden the new host country for the EMA...Many pharmaceutical executives also see a move as inevitable and they fear that a British "Out" vote would disrupt healthcare regulation in the world's biggest trading bloc...
- FDA Settles With Drugmaker in Fish-Oil Drug Marketing Case (abcnews.go.com)FDA deal with Amarin is unlikely to spark more off-label promotion (statnews.com)
The maker of a prescription fish-oil drug says it has reached a legal settlement that will allow it to promote unapproved uses of its drug for lowering fat levels...The closely watched case between Amarin and the Food and Drug Administration could strengthen the drug industry's hand in the ongoing debate over promoting drugs for uses that have not been declared safe and effective by regulators...FDA said...the settlement is "specific to this particular case and situation," and did not mark a new legal precedent...pharmaceutical experts said companies would likely pursue more aggressive legal action against FDA, in light of the settlement...We would expect companies throughout the country to ask courts to provide the same legal reasoning...Amarin won a surprise victory over the FDA when a U.S. District Court judge ruled that the company had a First Amendment right to distribute journal articles about unapproved indications for Vascepa (icosapent)...Drugmakers are not allowed to advertise drugs for "off-label" uses, or those that have not been cleared by the FDA as safe and effective. But companies' ability to distribute independent materials about their drugs — such as medical journal articles — has been subject to years of legal debate centering around the limits of "commercial speech."
- New FDA guidelines aim to prevent Zika transmission via tissue, cell donation (reuters.com)FDA issues recommendations to reduce the risk of Zika virus transmission by human cell and tissue products (fda.gov)
Food and Drug Administration on Tuesday issued new recommendations aimed at reducing the risk of Zika virus transmission through donated human tissues and cells used in surgical or reproductive procedures, such as umbilical cord blood, corneas and heart valves...The guidance is part of the agency's ongoing efforts to protect human cellular, tissue and blood products from potential contamination with Zika virus..."Though there is more to be learned about the transmission of Zika virus, given what we know about the virus at this point, which also is informed by our understanding of similar viruses, we must address the potential risk of Zika virus transmission by human cells and tissues," Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement...Under the new recommendations, donors should be considered ineligible if they were diagnosed with Zika virus infection or were in an area with active virus transmission, or had sex with a male with either of those risk factors, within the past six months...
- Senate confirms Dr. Robert Califf to lead FDA (reuters.com) New FDA head Robert Califf vows to use ‘bully pulpit,’ better explain agency decisions (washingtonpost.com)
The Senate voted overwhelmingly...to confirm Dr. Robert Califf as head of the Food and Drug Administration, an agency that regulates everything from food and drugs to tobacco, cosmetics and dietary supplements...Califf...a well-regarded cardiologist and researcher, takes the helm at the FDA when lawmakers are pressuring it to speed the approval process for drugs and medical devices and to finalize a proposed rule giving it authority to regulate e-cigarettes...He said one of his first priorities is to strengthen the workforce by reaching out to academic and other centers to attract new talent...Another priority...is improving surveillance systems to monitor for safety...We're not proposing to do away with the adverse event reporting system that currently exists...but we are acutely aware that it is not enough...Tools to monitor the safety of medical devices also need to be modernized...and though it will not happen overnight...we have to do the hard work of making it happen...