- 2015: A banner year for personalized medicine (catalyst.phrma.org)
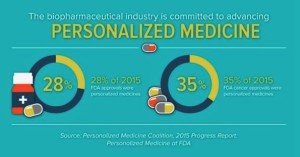
2015 was a record year for personalized medicine approvals, according to a new analysis from the Personalized Medicine Coalition. This news confirms the growing role of personalized medicine as an approach to treatment that can improve outcomes for patients and also create important efficiencies in the health care system. Personalized medicine is an emerging field of medicine that uses diagnostic tools to identify specific biological markers to help assess which medical treatments and procedures will be best for each patient. Personalized medicine also takes into account patients’ medical history, circumstances and values in developing targeted treatment and prevention plans...45 novel new drugs approved in 2015, the new analysis indicates that 28 percent of novel new drugs approved by the Food and Drug Administration...were personalized, or precision, medicines...Some of the personalized medicine highlights from 2015 include:
- Two new medicines for patients with different forms of non-small cell lung cancer;
- A new combination therapy for patients with cystic fibrosis;
- Two new medicines to help patients with a difficult-to-treat form of high cholesterol; and
- A new targeted therapy for melanoma.
- Senate Panel Punts on Big Medical Bill (morningconsult.com)
The Senate will not put forth a comprehensive medical innovation bill that would be a companion measure to the House’s 21st Century Cures bill. Instead, lawmakers are opting to work on several smaller bills that have bipartisan support. In recent weeks, aides said committee members hit partisan snags when discussing a bigger bill...The Health, Education, Labor, and Pensions Committee will hold three separate markups, deliberating a few “easier” bills in February to “get our sea legs on working on bipartisan FDA/NIH bills,” according to a senior GOP committee aide. The overall goal of the committee’s work will be to accelerate the development and approval of new medical cures...The House passed its 21st Century Cures bill last summer. Energy and Commerce Chairman Fred Upton guided the bill to a 344-77 passage on the floor, but HELP Committee Chairman Lamar Alexander has long said the Senate would produce its own version...Both chambers’ goals overlap, including giving the National Institutes of Health additional funding and reforming drug approvals at the Food and Drug Administration.
- FDA faulted for failure to track safety issues with drugs already on market (statnews.com)
Most Americans assume that drugs approved by the Food and Drug Administration are safe to take as directed. But safety concerns often arise only after the drugs go on the market, when companies or doctors tell the FDA about cases of patients who have fallen ill or died from their medications...a federal watchdog agency said the FDA is failing to sufficiently track and publicly disclose instances in such cases...Government Accountability Office investigation...raises...concerns about the FDA’s oversight. It expresses particular concern about the lack of tracking of drugs cleared under two expedited approval programs, which account for about one-quarter of all medicines permitted to go on the market...investigators also criticized the FDA for failing to post quarterly reports listing certain potential safety issues that it has identified. Despite a statutory requirement that it do so, last year FDA posted no reports at all in its tracking system...FDA lacks fundamental resources and leadership in ensuring that drugs brought quickly to market are truly safe and effective,”...“If FDA is shifting more of the safety risk to consumers by allowing fewer and shorter clinical trials on expedited drugs, adequate tracking of drug safety issues and review of post market studies are absolutely vital.”...The backlog of postmarket data has been a recurring problem at the FDA...
- FDA sues to stop a wayward drug compounder (statnews.com)
After nearly three years of sparring with a recalcitrant compounder, the Food and Drug Administration has filed a lawsuit asking a federal court to prevent Downing Labs from continuing operations. And the compounder agreed to a consent decree, which requires the company to take various steps before operations can resume...In the lawsuit, the agency cited numerous violations of so-called good manufacturing practices and several issued warnings to the company...about its failure to comply with regulations. Most recently, the Dallas-based compounder, which in 2014 refused to an FDA request to recall some products, failed yet another FDA inspection...the FDA inspectors found unsanitary conditions, according to the lawsuit...tests...found traces of...bacteria which, “if introduced into the body, can cause septic shock, pneumonia, and urinary tract infections,” the lawsuit stated...the Drug Quality and Security Act was passed to, in part, bolster compounding oversight. In fact, the FDA cited the defiant posture taken by Downing as an example of why a new law was needed to allow the agency to bolster its oversight and pursue legal options when compounders refused to upgrade operations.
- Licorice Coughing Liquid Recall, Presence of Morphine (infozine.com)Master Herbs, Inc. Issues Voluntary Nationwide Recall of All Lots of Licorice Coughing Liquid Due to the Presence of Morphine (fda.gov)
Master Herbs, Inc. (Ma Ying Long Pharmaceutical Group) is voluntarily recalling ALL LOTS of Licorice Coughing Liquid, cough syrup in 100 ml bottles to the consumer level. This product has been found to contain morphine, which is an opioid, and it is not declared on the label. Opioid is an ingredient of Compound Camphor. Compound Camphor is declared on the label of the product, but not its ingredients...The product is used for the temporary relief of cough due to cold, minor throat and bronchial irritations...identified by the Chinese Product Name: Licorice Coughing Liquid The product was distributed to Chinese grocery stores in various cities in California, New Jersey, Hawaii, Illinois, Ohio and Nevada.
- Supreme Court declines to hear J&J appeal of Children’s Motrin lawsuit (statnews.com)
In a setback to the pharmaceutical industry, the US Supreme Court declined on Tuesday to hear an appeal sought by Johnson & Johnson of a $63 million verdict that found the company failed to properly warn consumers about the risks of its Children’s Motrin painkiller...At issue was whether J&J should have upgraded its product labeling to reflect the risk that a patient may develop toxic epidermal necrolysis, which can lead to a rare disease called Stevens-Johnson syndrome...J&J sought to convince the Supreme Court that federal law preempted the state court verdict. In response to a citizen’s petition seeking upgraded labeling, the Food and Drug Administration had agreed that an increased warning about skin reactions — such as rashes and blisters — was warranted. But the agency did not agree to add the names of the skin diseases…the company maintained it would have violated federal law if the Motrin labeling was updated with the sort of specific language the family believed should have been used, according to its filing with the Supreme Court...J&J complained it was in an untenable bind…J&J, which now faces a $140 million payout when including interest...is “disappointed the Supreme Court declined to hear this case because we believe it raises important and unsettled preemption issues.”
- Illumina Launches New Company To Develop Gene-Based Blood Test To Detect Early-Stage Cancers (ibtimes.com)A revolutionary blood test that can detect cancer Liquid biopsies: A $20 billion market ready to explode. (cnbc.com)
Illumina, the world’s largest manufacturer of DNA sequencing machines, announced Sunday the formation of a new company that will develop blood tests that can detect a broad variety of early stage cancers long before symptoms arise. The new company, named Grail, has so far raised $100 million, mostly from Illumina and venture capital firm Arch Venture Partners, but also from Microsoft co-founder Bill Gates, and Amazon founder Jeff Bezos...The holy grail in oncology has been the search for biomarkers that could reliably signal the presence of cancer at an early stage...Illumina’s sequencing technology now allows the detection of circulating nucleic acids originating in the cancer cells themselves, a superior approach that provides a direct rather than surrogate measurement...We hope today is a turning point in the war on cancer...By enabling the early detection of cancer in asymptomatic individuals through a simple blood screen, we aim to massively decrease cancer mortality by detecting the disease at a curable stage...
- Express Scripts sued by compounding pharmacies for alleged antitrust practices (statnews.com)
For the second time since Express Scripts began blocking coverage of hundreds of ingredients used to make compounded medicines, several compounding pharmacies have filed a lawsuit accusing the pharmacy benefits manager of using illegal tactics...In the latest instance, a half-dozen compounding pharmacies have charged Express Scripts with violating antitrust laws and is attempting to force them out of business...Express Scripts has taken “a series of unreasonable restrictions and rules that would make it impossible for [the compounding pharmacies] to fill prescriptions” for patients “and obtain reimbursements that would cover their costs,”...The company and the other benefits managers “employed tactics designed to ensure that the compounding pharmacy industry …cannot survive.”...The move to cut back on covered ingredients has riled compounding pharmacies...the Food and Drug Administration has cracked down on compounding pharmacies by increasing the number of inspections, and, in rare cases, taking legal action to halt allegedly unsafe practices. The justifiable emphasis on safety has forced many compounding pharmacies to enhance operations...The compounding pharmacies are striking back. In November 2014, three others filed a lawsuit claiming Express Scripts illegally blocked legitimate prescriptions and unfairly forced patients to seek more expensive medicines or simply not seek treatment. The pharmacies maintained the benefits manager violated federal law because it lacks authority to essentially alter terms of health plans.
- FDA Takes Action Against Medical Device Hacking (newsmax.com)Postmarket Management of Cybersecurity in Medical Devices (fda.gov)
Food and Drug Administration on Friday issued draft guidelines to medical device makers on how to protect patients from cybersecurity vulnerabilities in the devices...Cybersecurity threats to medical devices are a growing concern...The exploitation of cybersecurity vulnerabilities presents a potential risk to the safety and effectiveness of medical devices...The draft guidance, which is not legally binding, recommends companies take a number of actions, including monitoring and assessing risk, adopting a coordinated vulnerability disclosure policy, and taking measures to address cybersecurity risk early.
- JPMorgan’s big health-care confab: What to expect (cnbc.com)What to watch at J.P. Morgan Health Care Conference (video.cnbc.com)
Exhausted. Depressed. These are the words biotech analysts and investors are using to describe their moods coming out of 2015. Which means this year's JPMorgan Health Care Conference, which kicks off next week, could take on a more muted tone than in previous years...Thousands of investors, analysts, executives and entrepreneurs head to San Francisco...for the conference, considered a barometer of sentiment across the industry as the year gets underway. More than 450 companies are slated to present to investors at the meeting...Sentiment will be weary, but not funeralesque...Normally, sentiment is extremely bullish at JPMorgan, but once every few years you get a situation like this...a 23 percent decline in biotech stocks from highs in July, driven by concerns over pressure on drug prices, valuations that have been rising for six years, and some stock-crushing clinical trial setbacks toward the end of the year. The Nasdaq biotechnology index sank 9.4 percent this week through Thursday…Despite the somber mood, 2015 wasn't as bad as it sounds. The Food and Drug Administration approved 45 new medicines, the most in 19 years. Deal activity was explosive, at more than 530 transactions worth more than $296 billion, according to MergerMarket, up 29 percent from 2014...It was also the sixth-straight year biotech outperformed the broader market...