- Gottlieb Spells out Vision for FDA Modernization Efforts to Create a new Data Enterprise (raps.org)
As the US Food and Drug Administration continues to navigate a bumpy road toward modernization, a new data enterprise will aim to enhance regulatory decisions on products...The growing number of novel medical products in the US prompted FDA to “refashion” the approaches to drug and device regulations and “create more modern platforms that are better suited to the efficient evaluation of these advances,” said FDA Commissioner Scott Gottlieb...The initiatives already underway...will help “fund the creation of a cross-cutting data enterprise for the generation of evidence, and a more modern and integrated approach to the evaluation of this information,”...Modernization is intended to make regulatory premarket reviews more consistent and predictable to ultimately increase competition among firms and allow for more timely patient access to a greater number of drugs and devices in the US market that are more affordable and of higher quality. This culture shift could also lead to reduced burden and potential cost savings...
- China drug scandals highlight risks to global supply chain (cnbc.com)
The drug safety scandals...have underlined the risks to international consumers posed by weak oversight in China, the world's largest supplier of active pharmaceutical ingredients...The European Medicines Agency and the US Food and Drug Administration issued alerts over a cancer-causing ingredient used in a blood pressure medication, supplied by Chinese company Zhejiang Huahai, resulting in a recall of affected drugs...Then Beijing announced that hundreds of thousands of substandard vaccine doses had been sold in China, prompting a public outcry. Senior executives were arrested at the pharma company, Changsheng Biotech, which was also accused by authorities of forging data during the production of rabies vaccines...China is home to thousands of API producers, with exports worth $29bn last year...its producers supply ingredients for generic drugmakers such as Teva Pharmaceutical and multinationals including Johnson & Johnson and Novartis. About 80 percent of APIs used in the US come from China and India…Warnings to Chinese companies published by the FDA and EMA in recent years show that dozens have violated standards, mainly relating to record-keeping during the manufacturing process. In several cases the exporters shipped large volumes of product before the infractions were discovered...
- Blood Pressure Medicine Is Recalled (nytimes.com)
The Food and Drug Administration has announced a voluntary recall of a widely prescribed blood pressure medication made in China, reviving fears about the safety of imported drugs...Three companies that sell the generic drug, valsartan, in the United States agreed to recall it after the F.D.A. said it might be tainted by N-nitrosodimethylamine (NDMA), considered a probable human carcinogen. The agency is still investigating, but said the contamination was believed to be related to changes in the way that valsartan was manufactured...All of the valsartan that is being recalled was made in China by the same company, Zhejiang Huahai Pharmaceutical Co. Ltd. It is distributed in the United States by three companies: Major Pharmaceuticals; Teva Pharmaceutical Industries, Ltd.; and Solco Healthcare... Other companies that market the drug, not subject to the recall, are Sun Pharma, Mylan, Jubiliant, Aurobindo and Hetero...The safety of imported drugs has long been debated. The F.D.A. said it would continue to investigate the levels of NDMA in the recalled products, determine the possible effect on patients who have been taking them, and assess what measures can be taken to reduce or eliminate the impurity from future batches...
- U.S. Health Regulators OK Marijuana-Based Drug for Seizures (ktvn.com)
The Food and Drug Administration approved the medication, called Epidiolex (cannabidiol), to treat two rare forms of epilepsy (Lennox-Gastaut Syndrome and Dravet Syndrome) that begin in childhood. But it's not quite medical marijuana...British drugmaker GW Pharmaceuticals studied the drug in more than 500 children and adults with hard-to-treat seizures, overcoming numerous legal hurdles that have long stymied research into cannabis...FDA officials said the drug reduced seizures when combined with older epilepsy drugs...The FDA has previously approved synthetic versions of another cannabis ingredient for medical use, including severe weight loss in patients with HIV...Epidiolex is essentially a pharmaceutical-grade version CBD oil, which some parents already use to treat children with epilepsy. CBD is one of more than 100 chemicals found in marijuana...it doesn't contain THC, the ingredient that gives marijuana its mind-altering effect...Physicians say it's important to have a consistent, government-regulated version...
- FDA approves first generic of Mylan’s EpiPen (pharmaceutical-technology.com)
The Food and Drug Administration has approved the first generic version of the EpiPen and EpiPen Jr auto-injector for severe allergic attacks, including life-threatening anaphylaxis....The EpiPen was approved in 2007 and is marketed by Mylan. However, since then both the company and its product have been involved in a number of scandals. Notably the price of the EpiPen has increased from $57 in 2007 to approximately $600 in 2016 and in May, the FDA had to place Mylan’s EpiPen on its official shortages list as a result of manufacturing delays...The newly approved generic epinephrine auto-injector will be available in 0.3mg and 0.15mg strengths and is marketed by Teva. This the company’s second attempt to get its generic approved by the FDA; it was rejected in 2016...FDA commissioner Scott Gottlieb said: “Today’s approval of the first generic version of the most-widely prescribed epinephrine auto-injector in the US is part of our longstanding commitment to advance access to lower cost, safe and effective generic alternatives once patents and other exclusivities no longer prevent approval.
- FDA seals loophole that allowed some drugmakers to avoid pediatric clinical trials (medcitynews.com)
PREA loophole had allowed companies to receive orphan drug designations for diseases common in adults...The Food and Drug Administration moved...to close a loophole that had inadvertently excused some drugmakers from having to conduct clinical trials in children for drugs to treat diseases that commonly affect adults...the FDA announced that it had finalized a draft guidance...intended to close a loophole in the Pediatric Research Equity Act that allowed drug companies making medicines for non-orphan diseases in adults – meaning those affecting more than 200,000 patients – to get pediatric subpopulation designation. Consequently, companies were exempt from conducting the clinical trials in children that PREA would normally have required...“Addressing the inadequate testing of drugs in pediatric populations has been a priority for the FDA, the medical community and Congress and has led to important laws to ensure this important, vulnerable population is not overlooked,” FDA Commissioner Scott Gottlieb said...
- Evidence to support ‘breakthrough’ drugs often very limited: study (reuters.com)
The 46 medicines given approval through 2017 as part of the Food and Drug Administration’s Breakthrough Therapy program have often been sent to patients without a large double-blind study, direct measurement of benefit, or comparison with a placebo or existing treatment, according to a new analysis...“FDA approval of these breakthrough therapies is generally based on shorter and smaller clinical trials than those that support FDA approval of non-breakthrough therapy drugs,” coauthor Dr. Joseph Ross....expediting drug approvals raises concerns that important safety or effectiveness information will be missed, potentially heightening risk of patient harm...The study, published in the Journal of the American Medical Association, did not examine whether the drugs were actually as effective and safe as pre-approval testing suggested. In some cases the FDA doesn’t require such post-marketing studies for “breakthrough” drugs...people, when they hear ‘breakthrough designation,’ assume the ‘breakthrough’ is based on great clinical trial evidence but it’s actually more of an expectation that this is promising...My expectation is that this is what the public and clinicians (and Congress!) wants - more novel therapies coming to market as quickly as is reasonably possible, while still assuring drug safety and efficacy...questions persist on whether a larger trial will produce a different result, whether the benefit will fade as treatment continues, whether a drug will prove to be dangerous over the long term and whether a drug will really make a difference in long-term quality of life or survival...
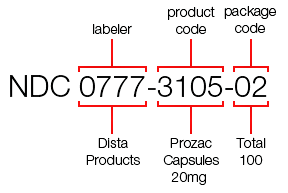
- FDA Anticipates Need for Expanding National Drug Code, Calls for Industry Input (raps.org)
The Food and Drug Administration announced...it plans to hold a public discussion on expanding the National Drug Code format and the impact on industry...FDA currently assigns 10-digit NDCs for the purposes of identifying drugs in the country and the format is comprised of three codes regarding the labeler, the product and the package...“FDA anticipates that it will run out of 5-digit labeler codes in approximately 15 years,” the agency wrote in the notice on the 5 November public hearing. “FDA will begin assigning 6-digit labeler codes at some point in the future due to exhaustion of 5-digit labeler codes.”...Prior to implementing the new formats for longer configurations, FDA officials will seek feedback from meeting participants on the potential impact on companies’ bottom line and drug safety, as well as “issues associated with the current lack of NDC uniformity in the marketplace.”
- Cooking Pots and Household Power Tools: FDA Warns California Drugmaker (raps.org)
Food and Drug Administration’s...warning letter for BioDiagnostic International featured an odd note about halfway down the CGMP violations: “You use kitchen cooking pots and household power tools to manufacture your drug product used for biopsy procedures.”...The surprising detail – part of one of four CGMP violations cited – comes as an FDA investigator further found an employee food prep station within its Brea, CA-based drug manufacturing area “with no separation between open manufacturing equipment, cooking utensils, and personal-use items.”...Parts of the facility to manufacture product...were also “open to the outdoors,” which FDA said increases “the likelihood of your drug products becoming contaminated.”...
- FDA to Consumers: Stay Away from Maximum Powerful (ptcommunity.com)
The Food and Drug Administration is advising consumers not to purchase or use Maximum Powerful, a product promoted for sexual enhancement that contains sildenafil, the active ingredient in Viagra. This product was identified during an examination of international mail shipments...The announcement is part of the agency’s effort to inform the public of a growing trend of dietary supplements or conventional foods with hidden drugs and chemicals. The culprit products are typically promoted for sexual enhancement, weight loss, and bodybuilding and are often represented as being “all natural.”...the FDA noted that it is unable to test and identify all products marketed as dietary supplements that have potentially harmful hidden ingredients. As a result, the agency issued a blanket warning that consumers should exercise caution before purchasing any product touted as improving sexual enhancement, helping with weight loss, or building up muscle...