- FDA staff flag likely dosing errors with Purdue’s opioid painkiller (reuters.com)
Food and Drug Administration…have expressed concerns over likely errors in administering Purdue Pharma's fast-acting oxycodone…(Avridi) that could result in inadequate relief…The drug is designed to be taken every 4-6 hours on an empty stomach…absorption…can be substantially delayed in the presence of food,.. "food effect" may reduce the effectiveness and safety of the drug,.. Inadequate pain control caused by presence of food could lead to overdosing…
- In a bad-news first for pharma reps, more than half of doctors now restrict access (fiercepharma.com)
It's no secret that pharma salespeople are barred at the doors of many doctors' offices. Those doors have been closing one by one for several years…barriers are rising at a bad time for drugmakers intent on launching new products to make up for patent-cliff losses. Just as doors are closing, there's been a renaissance in drug approvals, with the FDA blessing record numbers of meds in the past few years…pharma marketers have adopted workarounds--such as digital detailing--face-to-face meetings are still the cornerstone of new drug launches.
- Progress seen In addressing US drug shortages, but challenges remain (in-pharmatechnologist.com)
New US drug shortages this year are running roughly at the same levels as the last two years, and far below the record highs seen earlier this decade,…several significant products remain difficult to find, and hospitals, still shaken by the critical shortages of the recent past, remain concerned that key drugs could once again become scarce..
- Generic Drug Sponsors Will Get Status Reports– But Only For Old ANDAs (pharmamedtechbi.com)
FDA is ready to restore some informal communications for generic drugs, but only for applications not covered by the newly implemented user fee action dates…applicants with pending ANDAs (abbreviated new drug application) that are not covered by user-fee goals will have the right to a status report under a new policy …governing communications with ANDA applicants in response to widespread criticism from the generic industry about the loss of interaction that followed enactment of the Generic Drug User Fee law..
- Pfizer’s quit-smoking drug not linked to depression or heart risks (reuters.com)
Pfizer's stop-smoking drug Chantix (varenicline) does not raise risks of heart attack or depression, contrary to previous reports, and should be recommended to more smokers wanting to quit, scientists said…researchers found that patients who took Chantix,..marketed as Champix in Europe, were no more likely to suffer a heart attack than those using nicotine replacement therapy or another quit-smoking drug…also not at higher risk of depression or self-harm...
- Europe bans drugs tested by GVK; FDA monitors but keeps allowing sales (in-pharmatechnologist.com)
US FDA has found no systemic issues affecting the safety or efficacy of generics clinically tested at GVK BioSciences, but says it supports Europe’s ban of around 700 products…Last week, a European ban took effect on…medicines that were approved based …on what regulators called flawed clinical studies… US Food and Drug Administration isn’t taking any action on products sold here that included data from GVK’s studies in their applications,…
- 5 Big FDA Decisions Expected in September (247wallst.com)
Pharmaceutical companies usually are involved in a lengthy process in getting their drug candidates to market through clinical trials. There is a fair amount of risk involved,..should a study come back negative or should a candidate not be approved. Conversely, if a drug is approved or passes a clinical trial, there can be big upside. Food and Drug Administration rulings can make or break these companies…. big FDA decisions coming up on the calendar for the month of September..
- Opko Health - expecting a rolapitant PDUFA (Prescription Drug User Fee Act ) action date on September 5. (prevention of chemotherapy induced nausea and vomiting)
- Tesaro - tag along with Opko, has a New Drug Application for oral rolapitant under review by the FDA with the same PDUFA goal date.
- Zosano Pharma - completed its enrollment for the Phase 2 trial for ZP-Glucagon. (significant improvement over the currently marketed products for treatment of severe hypoglycemia)
- Xenoport - completed enrollment in its Phase 2 clinical trial of XP23829 as a potential treatment for moderate-to-severe chronic plaque-type psoriasis..
- Apricus Biosciences - enrolled its last patient in its RayVa Phase 2a proof-of-concept study, (Raynaud’s phenomenon secondary to scleroderma)
- FDA Grants Malignant Mesothelioma Treatment Orphan Drug Designation (specialtypharmacytimes.com)
Biologic therapy targets genetic defects found in various cancers… FDA granted Orphan Drug Designation to MTG-201 (MTG Biotherapeutics Inc.) for the treatment malignant mesothelioma…drug targets the Dickkopf-3 genetic defect found in various cancers…There is a…need for new treatment options for…mesothelioma…one of the most aggressive and poorly treated cancers. MTG-201 represents a…novel approach to treating this cancer by selectively inducing apoptosis and inducing an immunologic response against the cancer..
- Kim Kardashian Posts Drug Side Effects After FDA Warning (bloomberg.com)Kim Kardashian's FDA run-in shows the challenge of policing drug ads in the Instagram age (vox.com)
Kardashian's #CorrectiveAd came during MTV's Video Music Awards...Kim Kardashian West took to social media…to belatedly acknowledge the side effects of a controversial morning-sickness drug she endorses (Diclegis)… Aug. 7 warning from the U.S. Food and Drug Administration to drugmaker Duchesnay saying that Kardashian's original paid endorsement omitted important safety information.
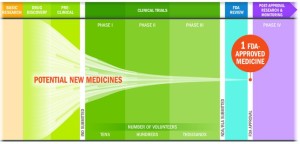
- Nine Explanations For Why The FDA Is Approving Almost Every New Drug Application (forbes.com)The drug development and approval process is about much more than the final “okay” (catalyst.phrma.org)
..Food and Drug Administration, which once approved as few as 40% of new drugs submitted by industry, has been on a green-light-almost-everything jag, approving 89% of drug applications. What’s more, a closer look showed an even higher approval rate. This year so far, 96% of new molecular entities.. – that have been submitted to the FDA have reached the market. For anyone who was watching the FDA a decade ago, that’s just shocking. Good or bad, it’s a radical change...there are a lot of factors that explain why the FDA approval rate is suddenly so high,..
- The approval rate is much lower, because only 12% of drugs that enter clinical trials reach the market.
- Drug companies are better at research, and they are simply producing better drugs.
- Drug companies are picking areas where the chances of approval are higher.
- The FDA is doing a better job communicating with companies.
- The FDA has more power to restrict the use of an approved drug than it used to.
- The FDA is taking a risk by taking strong stands against drug approvals right now.
- The FDA is without a permanent commissioner.
- It’s just random chance.
- In the current political environment, the agency is approving drugs it shouldn’t.