- Cities, counties and schools sidestep FDA foreign drug crackdown, saving millions (washingtonpost.com)
Schenectady County, N.Y., is on track to pay 20 percent less on prescription drugs for its employees this year than in 2003...Flagler County, Fla., expects to save nearly $200,000 in 2017 on brand-name medicines for its 800 workers, thanks to drug costs that have fallen 10 percent since 2016...dozens of cities, counties and school districts have found a solution they say protects their budgets and saves workers money: They are helping employees buy medicines from Canada and overseas, where prices are up to 80 percent cheaper...“We love it. . . . It’s a win-win,” said Anita Stoker, benefits and wellness manager for Flagler County, which has a program enabling its employees to get drugs from pharmacies in Canada, Britain, Australia and New Zealand...The number of municipalities offering this benefit is growing, even though the Food and Drug Administration considers such drug importation to be illegal and this fall began stepping up enforcement against storefronts advertising the same service. In October it raided nine central Florida locations that helped a mostly senior population order drugs from pharmacies in other countries. Investigators warned the stores’ owners that they were operating illegally and could face fines or jail time...Drugs ordered from overseas often come with the same packaging as in the United States. CanaRx, based in Windsor, Ontario, and ElectRx, based in Detroit, say they vet pharmacies to ensure customers get the real product. Counties, cities and schools, plus an increasing number of private companies, contract with one of these businesses for online service.
- FDA Expands Generic Drug Priority Reviews (raps.org)
Talk of bringing down the price of pharmaceuticals often hinges on generic competition, and the US is seeing approvals of new generic drugs faster and more consistently than ever – a trend likely to continue...The progress comes as Food and Drug Administration Commissioner Scott Gottlieb...indicated that the agency will expand which abbreviated new drug applications will see priority reviews..."Earlier this year we made changes to how we prioritize the agency’s generic drug submissions. The goal was to prioritize the review of generic applications until the FDA has approved three generic versions of each particular drug," Gottlieb said in a statement. "Today we’re expanding this competition-focused policy to prioritize any application that can meet the FDA’s approval standards at the point when the 180-day exclusivity period expires on a first generic entrant to a branded medicine."...The shift could accelerate generic competitors to market more quickly and help bring down costs, and comes a day after the Federal Trade Commission held a workshop on drug competition.
- Is FDA Going Too Far in Enforcing Drug Compounding Law? (c.ymcdn.com)
...the FDA is overstepping its authority and jeopardizing patient access to compounded medications...The pharmacies say the Food and Drug Administration has misinterpreted Congress’s intent when it enacted the Drug Quality and Security Act (DQSA)...by not allowing office-use compounding...The pharmacies also are concerned that the FDA has issued no rules for compounding but only guidance documents, which aren’t legally enforceable...‘Mass confusion has resulted from the usage of guidance documents, and the FDA is definitely circumventing the Administrative Procedure Act (APA) in our opinion,’’ Cynthia Blankenship, executive vice president of the International Academy of Compounding Pharmacists (IACP)...The APA governs how federal agencies propose and establish regulations. IACP wants Congress to pass a bill (H.R. 2871) they say will fix these issues...Since the DQSA was enacted, the FDA has significantly..increased its inspections of drug compounding...facilities...The FDA has conducted more than 350 inspections of compounding pharmacies as of Nov. 27, 2016, and nearly 120 of those inspections were due to reports of serious adverse events or product quality issues...
- Continuous Manufacturing: Pfizer, Vertex, AstraZeneca and Others Weigh FDA Plans (raps.org)
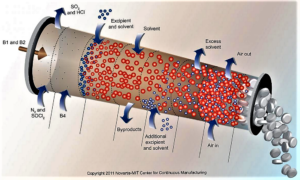
The US Food and Drug Administration has been encouraging the adoption of continuous manufacturing techniques...and several companies recently offered the agency some suggestions to refine its work around the developing technology...continuous manufacturing allows companies to move more seamlessly and efficiently...Last week, FDA finalized guidance on how manufacturers can participate in the agency’s program to advance continuous manufacturing...And in a blog post, Michael Kopcha, director of FDA's Office of Pharmaceutical Quality, pointed to Vertex's cystic fibrosis drug Orkambi (lumacaftor/ivacaftor) and Janssen's HIV treatment Prezista (darunavir) as examples of companies successfully using continuous manufacturing after engaging with FDA's emerging technology team...Vertex Pharmaceuticals...noted several contradictions in how batch size is described in an FDA document and sought further clarity and certainty regarding FDA’s understanding and expectations regarding batch size...AstraZeneca...asked if FDA might consider harmonizing the assessment process of the changes relating to continuous manufacturing, including a mechanism by which there could be some mutual recognition across countries participating in the International Council on Harmonization. AstraZeneca also said it "does not see the need" for a full ICH guideline on continuous manufacturing, which the company says FDA has been supporting. But the European drug industry group known as EFPIA is suggesting a Q&A document based on ICH Q8 and AstraZeneca says it "concurs with that approach."
- Massachusetts grabs spotlight by proposing new twist on Medicaid drug coverage (healthcarefinancenews.com)
Massachusetts' state Medicaid program hopes to road-test an idea both radical and market-driven. It wants the power to negotiate discounts for the drugs it purchases and to exclude drugs with limited treatment value..."This is a serious demonstration proposal,"..."They're not simply using [this idea] as an excuse to cut Medicaid. They're trying to take a step toward efficiency."...If the Department of Health and Human Services approves the...plan, others will likely take similar action...Currently, state Medicaid programs are required to cover almost all drugs that have received Food and Drug Administration approval, including multiple drugs from different manufacturers used for the same purpose and in the same category. In exchange, manufacturers must discount those drugs -- typically based on a set percentage of the list price...The idea is Medicaid's vulnerable beneficiaries get medications they need and the state doesn't go broke paying for them...Massachusetts wants to go a different route, requesting a federal exemption known as a Section 1115 waiver, which is meant to let states test ways of improving Medicaid. It wants to pick which drugs it covers based on most beneficiaries' medical needs and which medicines demonstrate the highest rates of cost effectiveness...It says it will be able to negotiate better prices as a result, saving public dollars while maintaining patients' access to needed therapies...
- FDA commissioner warns drug companies of ‘disruptive’ regulations to fight opioid epidemic (cnbc.com)
The Food and Drug Administration is likely to take new actions on opioids that may be "disruptive" and "uncomfortable" to drugmakers, the agency's commissioner (Scott Gottlieb) said...In addition to seeking to treat opioid-addicted patients with alternative medications that don't produce a high, the FDA says it will look at ways to reduce exposure to the drug. That includes new ways of packaging and distribution..."For example, it's possible that a defined, short-term supply of medication could be packaged in a manner that limits the number of pills dispensed,"..."We're at a point in this crisis that we're going to have to think of ideas and taking actions that are going to be more disruptive and are going to be uncomfortable to some parties," Gottlieb told "Squawk Box." "But we have to take more vigorous action to get ahead of this."...Gottlieb said the agency is having discussions with drug companies about the new packaging solutions..."Something like this could move potentially quickly," he said. "We're invested in taking a hard look at this and seeing what the opportunities are."...
- U.S. to promote use of opioid alternatives to treat addiction (reuters.com)
The U.S. Food and Drug Administration plans to encourage widespread use among opioid addicts of less harmful opioid drugs such as methadone and buprenorphine, a radical shift in policy that could draw opposition from those in the addiction field who believe abstinence is the only effective treatment...FDA Commissioner Scott Gottlieb outlined a proposal under which every addict who suffers a non-fatal overdose would be treated with an opioid substitute, for long periods if necessary, or even for life...“I know this may make some people uncomfortable,” Gottlieb said...“FDA will join efforts to break the stigma associated with medications used for addiction treatment.”...The FDA, Gottlieb said, will issue guidance for drugmakers to promote the development of new addiction treatments and lay out the agency’s interest in “novel, non-abstinence-based” products...
- FDA issues guidance that could make it easier for EpiPen rivals to come to market (cnbc.com)
When the controversy over the price of the EpiPen exploded late last summer, many consumers asked why there was no substitutable generic version available...The answer was complex: while the key ingredient in the anaphylaxis treatment, epinephrine, has been available for decades and is no longer covered by a patent, the delivery device proved hard for generic competitors to copy to a degree that would satisfy regulators...the Food and Drug Administration announced guidance seeking to change that, potentially streamlining a path to market for generic copies of complex medicines like the EpiPen and others...The FDA guidance says that generic copies with some design differences may be approved as substitutable products, as long as those differences don't affect patients' ability to use the product the way it's intended...Dr. Scott Gottlieb, said.."Under this guidance, so long as the generic applicant is able to demonstrate with data, where appropriate, that differences in design of the generic product do not affect the clinical effect or safety profile when the generic is substituted for the branded product, the generic product can be approved as a competitor to the branded drug where all other requirements for generic approval are met,"...
- FDA approves eight European regulators to inspect drug factories for U.S. (reuters.com)
The U.S. Food and Drug Administration said...it will allow regulators in eight European countries to determine whether drug manufacturing facilities there meet FDA requirements, freeing up American inspectors to spend more time in higher-risk countries like India and China...The FDA said it will begin relying on inspection data from the regulators in Austria, Croatia, France, Italy, Malta, Spain, Sweden and the United Kingdom as of Nov. 1...in some situations the United States could still do inspections in those countries...The move is the first step by the FDA to implement an agreement the United States and the EU finalized in March. The European Commission already determined in June that the FDA could carry out inspections for it in the United States...FDA Commissioner Scott Gottlieb said in a statement that partnering with European regulators would allow the agencies to get “the greatest bang for our collective inspectional buck.”
- Cancer drug study data was falsified, says AstraZeneca (telegraph.co.uk)
An early lab study supporting a cancer drug bought by British drugmaker AstraZeneca was falsified, the company has admitted...AstraZeneca bought a majority stake in US rival Acerta Pharma at the end of 2015 for $4bn on the strength of its novel drug acalabrutinib under development for treating blood cancers and solid tumours. More than 2,000 patients are taking part in more than 25 acalabrutinib clinical trials...Last month Acerta retracted an abstract, published in medical journal Cancer Research in August 2015 – four months before the AstraZeneca investment – purportedly showing the drug was effective in treating solid tumours in mice...AstraZeneca admitted this evidence had been fabricated. It blamed a “former Acerta employee who acted alone to falsify a pre-clinical data set provided through external collaborations”. It confirmed the incident pre-dated its investment and US medicines regulator the Food and Drug Administration had been notified...AstraZeneca added: “It’s important to note that this isolated issue had no impact on the integrity of acalabrutinib data in any clinical trials, and there was no risk to patient health.”...